Coenzyme Q10
Contents
Summary
- Coenzyme Q10 is a fat-soluble compound that is synthesized by the body and can be obtained from the diet. (More information)
- Coenzyme Q10 plays a central role in mitochondrial oxidative phosphorylation and the production of adenosine triphosphate (ATP). It also functions as an antioxidant in cell membranes and lipoproteins. (More information)
- Endogenous synthesis and dietary intake provide sufficient coenzyme Q10 to prevent deficiency in healthy people, although coenzyme Q10 concentrations in tissues decline with age. (More information)
- Oral supplementation of coenzyme Q10 increases coenzyme Q10 concentrations in plasma and lipoproteins, but it is unclear whether concentrations in peripheral tissues are increased, especially in healthy individuals. (More information)
- Oral high-dose coenzyme Q10 is usually effective to treat mitochondrial disorders that are caused by mutations in coenzyme Q10 biosynthetic genes. (More information)
- There is some evidence to suggest that coenzyme Q10 supplementation may be a useful adjunct to conventional medical therapy for congestive heart failure and in patients undergoing coronary artery bypass graft surgery. (More information)
- There are currently no proven therapeutic benefits of coenzyme Q10 supplementation in diabetes mellitus, neurodegenerative diseases, inherited ataxias, or breast cancer. (More information)
- Coenzyme Q10 supplementation does not appear to improve athletic performance. (More information)
- Although coenzyme Q10 supplements are relatively safe, they may decrease the anticoagulant efficacy of warfarin. (More information)
- The use of cholesterol-lowering medications called statins can decrease circulating coenzyme Q10 concentrations. However, there is no evidence that this causes any adverse side effects in statin-treated patients. (More information)
Introduction
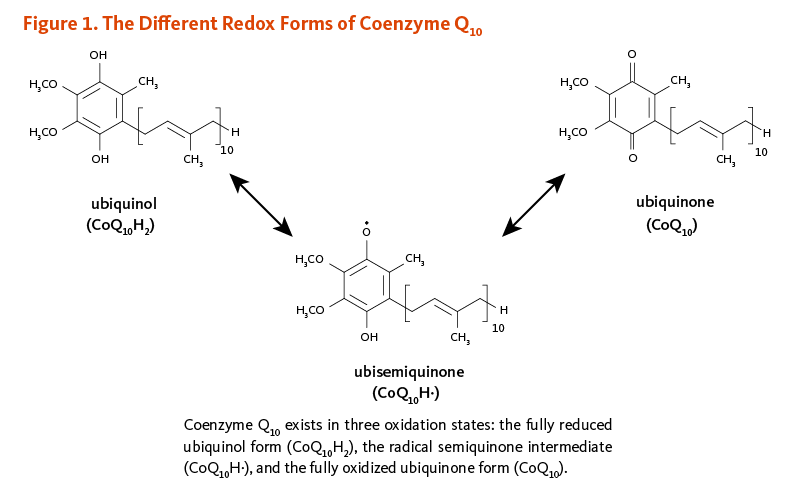
Coenzyme Q10 is a member of the ubiquinone family of compounds. All animals, including humans, can synthesize ubiquinones, hence, coenzyme Q10 is not considered a vitamin (1). The name ubiquinone refers to the ubiquitous presence of these compounds in living organisms and their chemical structure, which contains a functional group known as a benzoquinone. Ubiquinones are fat-soluble molecules with anywhere from 1 to 12 isoprene (5-carbon) units. The ubiquinone found in humans, ubidecaquinone or coenzyme Q10, has a "tail" of 10 isoprene units (a total of 50 carbon atoms) attached to its benzoquinone "head" (Figure 1) (1).
Biological Activities
Coenzyme Q10 is soluble in lipids (fats) and is found in virtually all cell membranes, including mitochondrial membranes. The ability of the benzoquinone head group of coenzyme Q10 to accept and donate electrons is a critical feature to its function. Coenzyme Q10 can exist in three oxidation states (Figure 1): (i) the fully reduced ubiquinol form, CoQ10H2; (ii) the radical semiquinone intermediate, CoQ10H·; and (iii) the fully oxidized ubiquinone form, CoQ10.
Mitochondrial ATP synthesis
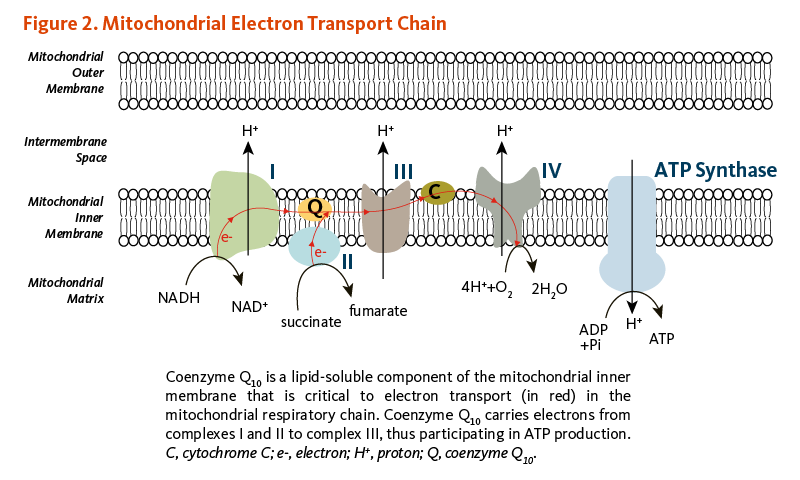
The conversion of energy from carbohydrates and fats to ATP, the form of energy used by cells, requires the presence of coenzyme Q10 in the inner mitochondrial membrane. As part of the mitochondrial electron transport chain, coenzyme Q10 accepts electrons from reducing equivalents generated during fatty acid and glucose metabolism and then transfers them to electron acceptors. At the same time, coenzyme Q10 contributes to transfer protons (H+) from the mitochondrial matrix to the intermembrane space, creating a proton gradient across the inner mitochondrial membrane. The energy released when the protons flow back into the mitochondrial interior is used to form ATP (Figure 2) (1). In addition to its role in ATP synthesis, mitochondrial coenzyme Q10 mediates the oxidation of dihydroorotate to orotate in the de novo pyrimidine synthesis.
Lysosomal function
Lysosomes are organelles within cells that are specialized for the digestion of cellular debris. The digestive enzymes within lysosomes function optimally at an acidic pH, meaning they require a permanent supply of protons. The lysosomal membranes that separate those digestive enzymes from the rest of the cell contain relatively high concentrations of coenzyme Q10. Research suggests that coenzyme Q10 plays an important role in the transport of protons across lysosomal membranes to maintain the optimal pH (2, 3).
Antioxidant functions
In its reduced form (CoQ10H2), coenzyme Q10 is an effective fat-soluble antioxidant that protects cell membranes and lipoproteins from oxidation. The presence of a significant amount of CoQ10H2 in cell membranes, along with enzymes capable of reducing oxidized CoQ10 back to CoQ10H2 (i.e., NAD(P)H oxidoreductases), supports the idea that CoQ10H2 is an important cellular antioxidant (4). CoQ10H2 has been found to inhibit lipid peroxidation when cell membranes and low-density lipoproteins (LDL) are exposed to oxidizing conditions. When LDL is oxidized, CoQ10H2 is the first antioxidant consumed. In isolated mitochondria, coenzyme Q10 can protect membrane proteins and mitochondrial DNA from the oxidative damage that accompanies lipid peroxidation (5). Moreover, when present, CoQ10H2 was found to limit the formation of oxidized lipids and the consumption of α-tocopherol (a form of vitamin E with antioxidant properties) (6). Indeed, in addition to neutralizing free radicals directly, CoQ10H2 is capable of regenerating antioxidants like α-tocopherol and ascorbate (vitamin C) (4). Finally, the role of coenzyme Q10 as an antioxidant is also exemplified by recent evidence showing that mitochondrial coenzyme Q10 deficiency causes an increased production of mitochondrial superoxide radical anion (O2•–) which might be driving insulin resistance in adipose and muscle tissues (7).
Nutrient interactions
Vitamin E
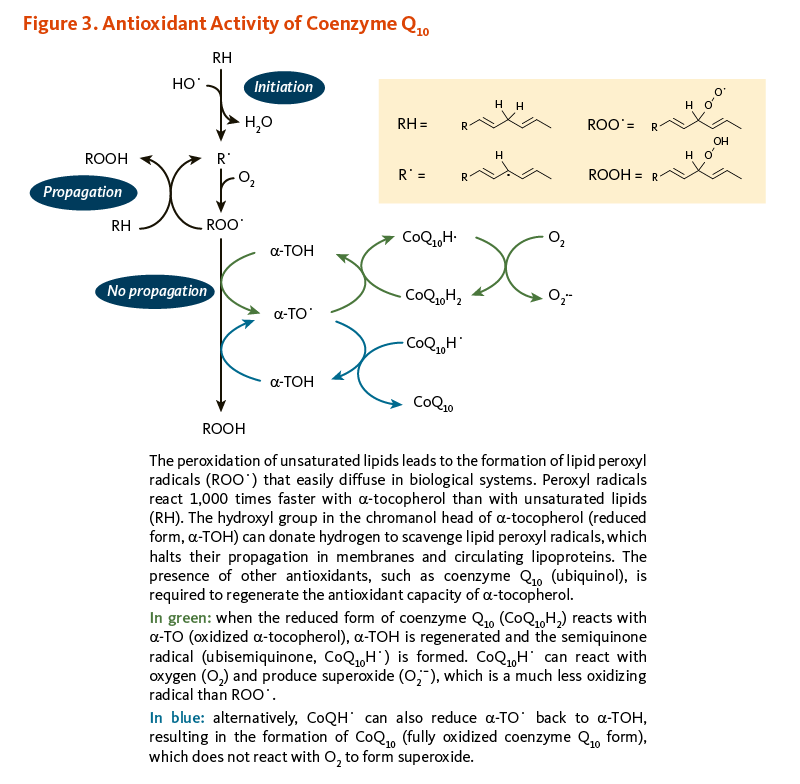
α-Tocopherol (vitamin E) and coenzyme Q10 are the principal fat-soluble antioxidants in membranes and lipoproteins. When α-tocopherol (α-TOH) neutralizes a free radical, such as a lipid peroxyl radical (LOO·), it becomes oxidized itself, forming α-TO·, which can in turn promote the oxidation of lipoproteins under certain conditions in the test tube, thus propagating a chain reaction. However, when the reduced form of coenzyme Q10 (CoQ10H2) reacts with α-TO·, α-TOH is regenerated and the semiquinone radical (CoQ10H·) is formed. It is possible for CoQ10H· to react with oxygen (O2) to produce superoxide anion radical (O2·-), which is a less reactive pro-oxidant than LOO·. However, CoQ10H· can also reduce α-TO· back to α-TOH, resulting in the formation of fully oxidized coenzyme Q10 (CoQ10), which does not react with O2 to form O2·- (Figure 3) (6, 8).
Deficiency
Coenzyme Q10 deficiency has not been described in the general population, so it is generally assumed that normal biosynthesis, with or without a varied diet, provides sufficient coenzyme Q10 to sustain energy production in healthy individuals (9).
Primary coenzyme Q10 deficiency is a rare genetic disorder caused by mutations in genes involved in coenzyme Q10 biosynthetic pathway. To date, mutations in at least nine of these genes have been identified (1). As a result, primary coenzyme Q10 deficiency is a clinically heterogeneous disorder that includes five major phenotypes: (i) severe infantile multi-systemic disease, (ii) encephalomyopathy, (iii) cerebellar ataxia, (iv) isolated myopathy, and (v) nephrotic syndrome. Whereas most mitochondrial respiratory chain disorders are hardly amenable to treatments, oral coenzyme Q10 supplementation has been shown to improve muscular symptoms in some (yet not all) patients with primary coenzyme Q10 deficiency (10). Neurological symptoms in patients with cerebellar ataxia are only partially relieved by coenzyme Q10 (CoQ10H2) supplementation (10).
Secondary coenzyme Q10 deficiency results from mutations or deletions in genes that are not directly related to coenzyme Q10 biosynthetic pathway. Evidence of secondary coenzyme Q10 deficiency has been reported in several mitochondrial disorders, such as mitochondrial DNA depletion syndrome, Kearns-Sayre syndrome, or multiple acyl-CoA dehydrogenase deficiency (MADD) (10). Secondary coenzyme Q10 deficiency has also been identified in non-mitochondrial disorders, such as cardiofaciocutaneous syndrome and Niemann-Pick-type C disease (11). Because the therapeutic potential of supplemental coenzyme Q10 is limited to its capacity to restore electron transfer in a defective mitochondrial respiratory chain and/or to increase antioxidant defense, patients with secondary coenzyme Q10 deficiency may fail to respond to supplementation (see Disease Treatment).
Coenzyme Q10 concentrations have been found to decline gradually with age in a number of different tissues (5, 12), but it is unclear whether this age-associated decline constitutes a deficiency (see Disease Prevention) (13). Decreased plasma concentrations of coenzyme Q10 have been observed in individuals with diabetes mellitus, cancer, and congestive heart failure (see Disease Treatment). Lipid-lowering medications that inhibit the activity of 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A (CoA) reductase (statins), a critical enzyme in both cholesterol and coenzyme Q10 biosynthesis, decrease plasma coenzyme Q10 concentrations (see HMG-CoA reductase inhibitors [statins]), although it remains unproven that this has any clinical implications.
Disease Prevention
Aging
According to the free radical and mitochondrial theories of aging, oxidative damage of cell structures by reactive oxygen species (ROS) plays an important role in the functional declines that accompany aging (14). ROS are generated by mitochondria as a byproduct of ATP production. If not neutralized by antioxidants, ROS may damage mitochondria over time, causing them to function less efficiently and to generate more damaging ROS in a self-perpetuating cycle. Coenzyme Q10 plays an important role in mitochondrial ATP synthesis and functions as an antioxidant in mitochondrial membranes (see Biological Activities). One of the hallmarks of aging is a decline in energy metabolism in many tissues, especially liver, heart, and skeletal muscle. Tissue concentrations of coenzyme Q10 have been found to decline with age, thereby accompanying age-related declines in energy metabolism (12). Early animal studies have not been able to demonstrate an effect of lifelong dietary supplementation with coenzyme Q10 on the lifespan of rats or mice (15-17). Nonetheless, more recent studies have suggested that supplemental coenzyme Q10 could promote mitochondrial biogenesis and respiration (18, 19) and delay senescence in transgenic mice (19). Presently, there is limited scientific evidence to suggest that coenzyme Q10 supplementation prolongs life or prevents age-related functional declines in humans. In a small randomized controlled trial, elderly individuals (>70 years) who received a combination of selenium (100 µg/day) and coenzyme Q10 (200 mg/day) for four years reported an improvement in vitality, physical performance, and quality of life (20). Further, a 12-year follow-up of these participants showed a reduction in cardiovascular mortality with supplemental selenium and coenzyme Q10 compared to placebo (21).
Atherosclerosis
Oxidative modification of low-density lipoproteins (LDL) in arterial walls is thought to represent an early event leading to the development of atherosclerosis. Reduced coenzyme Q10 (CoQ10H2) inhibits the oxidation of LDL in the test tube (in vitro) and works together with α-tocopherol (α-TOH) to inhibit LDL oxidation by regenerating α-TO· back to α-TOH. In the absence of a co-antioxidant, such as CoQ10H2 or vitamin C, α-TO· can, under certain conditions, promote the oxidation of LDL in vitro (6). Supplementation with coenzyme Q10 increases the concentration of CoQ10H2 in human LDL (22). Studies in apolipoprotein E-deficient mice, an animal model of atherosclerosis, found that coenzyme Q10 supplementation with supra-pharmacological amounts of coenzyme Q10 inhibited lipoprotein oxidation in the blood vessel wall and the formation of atherosclerotic lesions (23). Interestingly, co-supplementation of these mice with α-TOH and coenzyme Q10 was more effective in inhibiting atherosclerosis than supplementation with either α-TOH or coenzyme Q10 alone (24).
Another important step in the development of atherosclerosis is the recruitment of immune cells known as monocytes into the blood vessel walls. This recruitment is dependent in part on monocyte expression of cell adhesion molecules (integrins). Supplementation of 10 healthy men and women with 200 mg/day of coenzyme Q10 for 10 weeks resulted in significant decreases in monocyte expression of integrins, suggesting another potential mechanism for the inhibition of atherosclerosis by coenzyme Q10 (25). Although coenzyme Q10 supplementation shows promise as an inhibitor of LDL oxidation and atherosclerosis, more research is needed to determine whether coenzyme Q10 supplementation can inhibit the development or progression of atherosclerosis in humans.
Disease Treatment
Primary and secondary coenzyme Q10 deficiencies
Inherited coenzyme Q10 deficiencies are rare diseases that are clinically and genetically heterogeneous (see Deficiency). In individuals with primary coenzyme Q10 deficiency, early treatment with high-dose coenzyme Q10 supplementation (10–30 mg/kg/day in children and 1.2–3.0 g/day in adults) may improve the pathological phenotype, yet the effectiveness depends on the type of mutations affecting the coenzyme Q10 biosynthetic pathway (1, 26). Early treatment with pharmacological doses of coenzyme Q10 is essential to limit irreversible organ damage in coenzyme Q10-responsive deficiencies (1).
It is not clear to what extent coenzyme Q10 supplementation might have therapeutic benefit in patients with inherited secondary Q10 deficiencies. For example, multiple acyl-CoA dehydrogenase deficiency (MADD), caused by mutations in genes that impair the activity of enzymes involved in the transfer of electrons from acyl-CoA to coenzyme Q10, is usually responsive to riboflavin monotherapy yet patients with low coenzyme Q10 concentrations might also benefit from co-supplementation with coenzyme Q10 and riboflavin (27). Another study suggested clinical improvements in secondary coenzyme Q10 deficiency with supplemental coenzyme Q10 in patients presenting with ataxia (28). Because the cause of secondary coenzyme Q10 in inherited conditions is generally unknown, it is difficult to predict whether improving coenzyme Q10 status with supplemental coenzyme Q10 would lead to clinical benefits for the patients.
Finally, coenzyme Q10 deficiency can be secondary to the inhibition of HMG-CoA reductase by statin drugs (see Deficiency). A 2015 meta-analysis of six small, randomized controlled trials found no reduction in statin-induced muscle pain with 100 to 400 mg/day of supplemental coenzyme Q10 for one to three months (29). The trials failed to establish a diagnosis of relative coenzyme Q10 deficiency before the intervention started, hence limiting the conclusion of the meta-analysis. While statin therapy may not necessary lead to a reduction in circulating coenzyme Q10 concentrations, further research needs to examine whether secondary coenzyme Q10 deficiency might be predisposing patients to statin-induced myalgia (30).
Cardiovascular disease
Congestive heart failure
Impairment of the heart's ability to pump enough blood for all of the body's needs is known as congestive heart failure. In coronary heart disease (CHD), accumulation of atherosclerotic plaque in the coronary arteries may prevent parts of the cardiac muscle from getting adequate blood supply, ultimately resulting in heart damage and impaired pumping ability. Heart failure can also be caused by myocardial infarction, hypertension, diseases of the heart valves, cardiomyopathy, and congenital heart diseases. Because physical exercise increases the demand on the weakened heart, measures of exercise tolerance are frequently used to monitor the severity of heart failure. Echocardiography is also used to determine the left ventricular ejection fraction, an objective measure of the heart's pumping ability (31).
A study of 1,191 heart failure patients found that low plasma coenzyme Q10 concentration was a good biomarker of advanced heart disease (32). A number of small intervention trials that administered supplemental coenzyme Q10 to congestive heart failure patients have been conducted. A 2014 literature review identified seven small randomized controlled trials examining the effect of coenzyme Q10 supplementation (60-200 mg/day for ≤3 months in most trials) in heart failure patients (33). Pooling data from some of the trials showed an increase in serum coenzyme Q10 concentrations (three studies) but no effect on left ventricular ejection fraction (two studies) or exercise capacity (two studies) (33). However, a recent meta-analysis of 14 randomized, placebo-controlled trials in 2,149 participants with heart failure found that supplemental coenzyme Q10 (30-300 mg/day) resulted in a 39% reduction in mortality (seven studies), improved exercise capacity (four studies), but had no effect on left ventricular ejection fraction (nine studies) compared to placebo (34).
A trial is presently being conducted to assess the value of supplemental coenzyme Q10 and/or D-ribose in the treatment of congestive heart failure in patients with normal left ventricular ejection fraction (35).
Ischemia-reperfusion injury
The heart muscle may become oxygen-deprived (ischemic) as the result of myocardial infarction or during cardiac surgery. Increased generation of reactive oxygen species (ROS) when the heart muscle's oxygen supply is restored (reperfusion) might be an important contributor to myocardial damage occurring during ischemia-reperfusion (36). Pretreatment of animals with coenzyme Q10 has been found to preserve myocardial function following ischemia-reperfusion injury by increasing ATP concentration, enhancing antioxidant capacity and limiting oxidative damage, regulating autophagy, and reducing cardiomyocyte apoptosis (37). Another potential source of ischemia-reperfusion injury is aortic clamping during some types of cardiac surgery, such as coronary artery bypass graft (CABG) surgery. Early placebo-controlled trials found that coenzyme Q10 pretreatment (60-300 mg/day for 7-14 days prior to surgery) provided some benefit in short-term outcome measures after CABG surgery (38, 39). In a small randomized controlled trial in 30 patients, oral administration of coenzyme Q10 for 7 to 10 days before CABG surgery reduced the need for mediastinal drainage, platelet transfusion, and positive inotropic drugs (e.g. dopamine) and the risk of arrhythmia within 24 hours post-surgery (40). In one trial that did not find preoperative coenzyme Q10 supplementation to be of benefit, patients were treated with 600 mg of coenzyme Q10 12 hours prior to surgery (41), suggesting that preoperative coenzyme Q10 treatment may need to commence at least one week prior to CABG surgery to improve surgical outcomes. The combined administration of coenzyme Q10, lipoic acid, omega-3 fatty acids, magnesium orotate, and selenium at least two weeks before CABG surgery and four weeks after was examined in a randomized, placebo-controlled trial in 117 patients with heart failure (42). The treatment resulted in lower concentration of troponin-I (a marker of cardiac injury), shorter length of hospital stay, and reduced risk of postoperative transient cardiac dysfunction compared to placebo (42).
Although trials have included relatively few people and examined mostly short-term, post-surgical outcomes, the results are promising (43).
Periprocedural myocardial injury
Coronary angioplasty (also called percutaneous coronary intervention) is a nonsurgical procedure for treating obstructive coronary heart disease, including unstable angina pectoris, acute myocardial infarction, and multivessel coronary heart disease. Angioplasty involves temporarily inserting and inflating a tiny balloon into the clogged artery to help restore the blood flow to the heart. Periprocedural myocardial injury that occurs in up to one-third of patients undergoing otherwise uncomplicated angioplasty increases the risk of morbidity and mortality at follow-up.
A prospective cohort study followed 55 patients with acute ST segment elevation myocardial infarction (a type of heart attack characterized by the death of some myocardial tissue) who underwent angioplasty (44). Plasma coenzyme Q10 concentration one month after angioplasty was positively correlated with less inflammation and oxidative stress and predicted favorable left ventricular end-systolic volume remodeling at six months (44). One randomized controlled trial has examined the effect of coenzyme Q10 supplementation on periprocedural myocardial injury in patients undergoing coronary angioplasty (45). The administration of 300 mg of coenzyme Q10 12 hours before the angioplasty to 50 patients reduced the concentration of C-reactive protein ([CRP]; a marker of inflammation) within 24 hours following the procedure compared to placebo. However, there was no difference in concentrations of two markers of myocardial injury (creatine kinase and troponin-I) or in the incidence of major adverse cardiac events one month after angioplasty between active treatment and placebo (45). Additional trials are needed to examine whether coenzyme Q10 therapy can improve clinical outcomes in patients undergoing coronary angioplasty.
Angina pectoris
Myocardial ischemia may also lead to chest pain known as angina pectoris. People with angina pectoris often experience symptoms when the demand for oxygen exceeds the capacity of the coronary circulation to deliver it to the heart muscle, e.g., during exercise. Five small placebo-controlled studies have examined the effects of oral coenzyme Q10 supplementation (60-600 mg/day) in addition to conventional medical therapy in patients with chronic stable angina (46). In most of the studies, coenzyme Q10 supplementation improved exercise tolerance and reduced or delayed electrocardiographic changes associated with myocardial ischemia compared to placebo. However, only two of the studies found significant decreases in symptom frequency and use of nitroglycerin with coenzyme Q10 supplementation. Presently, there is only limited evidence suggesting that coenzyme Q10 supplementation would be a useful adjunct to conventional angina therapy.
Hypertension
Very few high-quality trials have examined the potential therapeutic benefit of coenzyme Q10 supplementation in the treatment of primary hypertension (47). A systematic review identified two small randomized, double-blind, placebo-controlled trials that found little evidence of a reduction in systolic or diastolic blood pressure following the administration of coenzyme Q10 (100-200 mg/day) for three months (47). In contrast, a meta-analysis that used less stringent selection criteria included 17 small trials and found evidence of a blood pressure-lowering effect of coenzyme Q10 in patients with cardiovascular disease or metabolic disorders (48). The effect of coenzyme Q10 on blood pressure needs to be examined in large, well-designed clinical trials.
Cardiovascular risk factors
Endothelial dysfunction: Normally functioning vascular endothelium promotes blood vessel relaxation (vasodilation) when needed (for example, during exercise) and inhibits the formation of blood clots. Atherosclerosis is associated with impairment of vascular endothelial function, thereby compromising vasodilation and normal blood flow. Endothelium-dependent vasodilation is impaired in individuals with elevated serum cholesterol concentrations, as well as in patients with coronary heart disease or diabetes mellitus. A 2012 meta-analysis examining the results of five small randomized controlled trials in 194 subjects in total found that supplemental coenzyme Q10 (150-300 mg/day for 4 to 12 weeks) resulted in a clinically significant, 1.7% increase in flow-dependent endothelial-mediated dilation (49). Evidence from larger studies is needed to further establish the effect of coenzyme Q10 on endothelium-dependent vasodilation.
Inflammation: Several small randomized controlled trials in patients at increased cardiovascular disease risk or with established cardiovascular disease have examined the effect of supplemental coenzyme Q10 for ≤3 months on circulating inflammation markers i.e., CRP, interleukin-6, and/or tumor necrosis factor-α. Recently published pooled analyses of these trials have given mixed results (50-52). Larger studies are needed to examine the effect of coenzyme Q10 supplementation on low-grade inflammation.
Blood lipids: Elevated plasma lipoprotein(a) concentration is an independent risk factor for cardiovascular disease. A meta-analysis of six controlled trials (of which five were randomized) in 409 participants found a reduction in plasma lipoprotein(a) concentration with coenzyme Q10 supplementation (100-300 mg/day for 4-12 weeks) (53). Other effects of coenzyme Q10 on blood lipids have not been reported (51, 53, 54).
A therapeutic approach combining coenzyme Q10 with other antioxidants might prove to be more effective to target co-existing metabolic disorders in individuals at risk for cardiovascular disease (55).
Diabetes mellitus
Diabetes mellitus is a condition of increased oxidative stress and impaired energy metabolism. Plasma concentrations of reduced coenzyme Q10 (CoQ10H2) have been found to be lower in diabetic patients than healthy controls after normalization to plasma cholesterol concentrations (56, 57). Randomized controlled trials that examined the effect of coenzyme Q10 supplementation found little evidence of benefits on glycemic control in patients with diabetes mellitus. A meta-analysis of six trials in participants with type 2 diabetes and one trial in participants with type 1 diabetes found that 100 to 200 mg/day of coenzyme Q10 for three to six months lowered neither fasting plasma glucose nor levels of glycated hemoglobin ([HbA1c]; a measure of glycemic control). Maternally inherited diabetes mellitus-deafness syndrome (MIDD) is caused by a mutation in mitochondrial DNA, which is inherited exclusively from one's mother. MIDD accounts for less than 1% of all cases of diabetes. Some early evidence suggested that long-term coenzyme Q10 supplementation (150 mg/day) may improve insulin secretion and prevent progressive hearing loss in these patients (58, 59).
Of note, the pathogenesis of type 2 diabetes mellitus involves the early onset of glucose intolerance and hyperinsulinemia associated with the progressive loss of tissue responsiveness to insulin. Recent experimental studies tied insulin resistance to a decrease in coenzyme Q10 expression and showed that supplementation with coenzyme Q10 could restore insulin sensitivity (7). Coenzyme Q10 supplementation might thus be a more useful tool for the primary prevention of type 2 diabetes rather than for its management.
Neurodegenerative diseases
Parkinson's disease
Parkinson's disease is a degenerative neurological disorder characterized by tremors, muscular rigidity, and slow movements. It is estimated to affect approximately 1% of Americans over the age of 65. Mitochondrial dysfunction and oxidative damage in a part of the brain called the substantia nigra may play a role in the development of the disease (60). Decreased ratios of reduced-to-oxidized coenzyme Q10 have been found in platelets of individuals with Parkinson's disease (61, 62). One study also found higher concentrations of oxidized coenzyme Q10 in the cerebrospinal fluid of patients with untreated Parkinson’s disease compared to healthy controls (63). Additionally, a study in postmortem Parkinson’s disease patients found lower concentrations of total coenzyme Q10 in the cortex region of the brain compared to age-matched controls, but no differences were seen in other brain areas, including the striatum, substantia nigra, and cerebellum (64).
A 16-month randomized, placebo-controlled phase II clinical trial evaluated the safety and efficacy of 300, 600, or 1,200 mg/day of coenzyme Q10 in 80 people with early Parkinson's disease (65). Coenzyme Q10 supplementation was well tolerated at all doses and resulted in a slower deterioration of function in Parkinson's disease patients in the group taking 1,200 mg/day. A phase III clinical trial was then designed to further examine the effect of high-dose coenzyme Q10 (1,200-2,400 mg/day) and vitamin E (1,200 IU/day) supplementation on both motor and non-motor symptoms associated with Parkinson’s disease. This trial was prematurely terminated because it was unlikely that such a treatment was effective in treating Parkinson’s disease (66). A smaller placebo-controlled trial showed that oral administration of 300 mg/day of coenzyme Q10 for 48 to 96 months moderately improved motor symptoms in treated patients (with Levodopa) with re-emerging symptoms but not in patients at an early stage of the disease (67). Two recent meta-analyses of randomized, placebo-controlled trials found no evidence that coenzyme Q10 improved motor-related symptoms or delayed the progression of the disease when compared to placebo (68, 69).
Huntington's disease
Huntington's disease is an inherited neurodegenerative disorder characterized by selective degeneration of nerve cells known as striatal spiny neurons. Symptoms, such as movement disorders and impaired cognitive function, typically develop in the fourth decade of life and progressively deteriorate over time. Animal models indicate that impaired mitochondrial function and glutamate-mediated neurotoxicity may be involved in the pathology of Huntington's disease. Some, but not all, studies in mouse models of Huntington’s disease have suggested that coenzyme Q10 supplementation could improve motor performance, overall survival, and various hallmarks of Huntington's disease, i.e., brain atrophy, ventricular enlargement, and striatal neuronal atrophy (70, 71). Interestingly, co-administration of coenzyme Q10 with remacemide (an NMDA receptor antagonist), the antibiotic minocycline, or creatine led to greater improvements in most biochemical and behavioral parameters (70-72).
To date, only two clinical trials have examined whether coenzyme Q10 might be efficacious in human patients with Huntington's disease. A 30-month, randomized, placebo-controlled trial of coenzyme Q10 (600 mg/day), remacemide, or both in 347 patients with early Huntington's disease found that neither coenzyme Q10 nor remacemide significantly altered the decline in total functional capacity, although coenzyme Q10 supplementation (with or without remacemide) resulted in a nonsignificant trend toward a slower decline (73). A 20-week pilot trial examined the safety and tolerability of increasing dosages of coenzyme Q10 (1,200 mg/day, 2,400 mg/day, and 3,600 mg/day) in eight healthy subjects and in 20 patients with Huntington’s disease; 22 of the subjects completed the study (74). All dosages were generally well tolerated, with gastrointestinal symptoms being the most frequently reported adverse effect. Blood concentrations of coenzyme Q10 at the end of the study were maximized with the daily dose of 2,400 mg (74). This dose was tested in a multicenter phase III clinical trial in 609 participants with early-stage Huntington’s disease. Participants were randomized to receive either 2,400 mg/day of coenzyme Q10 or placebo for five years (75). The trial was prematurely halted because it appeared unlikely to demonstrate any health benefit in supplemented patients — about one-third of participants completed the trial at the time of study termination (75). Although coenzyme Q10 is generally well tolerated, there is no evidence that supplementation can improve functional and cognitive symptoms in Huntington's disease patients.
Inherited ataxias
Friedreich's ataxia (FRDA): FRDA is an autosomal recessive neurodegenerative disease caused by mutations in the gene FXN that encodes for the mitochondrial protein, frataxin. Frataxin is needed for the making of iron-sulfur clusters (ISC). ISC-containing subunits are especially important for the mitochondrial respiratory chain and for the synthesis of heme-containing proteins (76). Frataxin deficiency is associated with imbalances in iron-sulfur containing proteins, mitochondrial respiratory chain dysfunction and lower ATP production, and accumulation of iron in the mitochondria, which increases oxidative stress and oxidative damage to macromolecules of the respiratory chain (77). Clinically, FRDA is a progressive disease characterized by ataxia, areflexia, speech disturbance (dysarthria), sensory loss, motor dysfunction, cardiomyopathy, diabetes, and scoliosis (77). A pilot study administering coenzyme Q10 (200 mg/day) and vitamin E (2,100 IU/day) to 10 FDRA patients found that energy metabolism of cardiac and skeletal muscle was improved after only three months of therapy (78). Follow-up assessments at 47 months indicated that cardiac and skeletal muscle improvements were maintained and that FRDA patients showed significant increases in fractional shortening, a measure of cardiac function. Moreover, the therapy was effective at preventing the progressive decline of neurological function (79). Another study reported both coenzyme Q10 and vitamin E deficiencies among FRDA patients and suggested that co-supplementation with both compounds, at doses as low as 30 mg/day of coenzyme Q10 and 4 IU/day of vitamin E, might improve disease symptoms (80). Large-scale, randomized controlled trials are necessary to determine whether coenzyme Q10, in conjunction with vitamin E, has therapeutic benefit in FRDA. At present, about one-half of patients use coenzyme Q10 and vitamin E supplements despite the lack of proven therapeutic benefit (77).
Spinocerebellar ataxias (SCAs): SCAs are a group of rare autosomal dominant neurodegenerative diseases characterized by gait difficulty, loss of hand dexterity, dysarthria, and cognitive decline. SCA1, 2, 3, and 6 are the most common SCAs (81). In vitro coenzyme Q10 treatment of forearm skin fibroblasts isolated from patients with SCA2 was found to reduce oxidative stress and normalize complex I and II-III activity of the mitochondrial respiratory chain (82). A multicenter prospective cohort study that followed 319 patients with SCAs (≥15 years) found no difference in the rate of disease progression over two years between those taking supplemental coenzyme Q10 (median dose, 600 mg/day) and nonusers (81).
Cancer
Early interest in coenzyme Q10 as a potential therapeutic agent in cancer was stimulated by an observational study that found that individuals with lung, pancreas, and especially breast cancer were more likely to have low plasma coenzyme Q10 concentrations than healthy controls (83). Two randomized controlled trials have explored the effect of coenzyme Q10 as an adjunct to conventional therapy for breast cancer. Supplementation with coenzyme Q10 failed to improve measures of fatigue and quality of life in patients newly diagnosed with breast cancer (84) and in patients receiving chemotherapy (85).
Performance
Athletic performance
There is little evidence that supplementation with coenzyme Q10 improves athletic performance in healthy individuals. A few placebo-controlled trials have examined the effects of 100 to 150 mg/day of supplemental coenzyme Q10 for three to eight weeks on physical performance in trained and untrained men. Most did not find significant differences between the group taking coenzyme Q10 and the group taking placebo with respect to measures of aerobic exercise performance, such as maximal oxygen consumption (VO2 max) and exercise time to exhaustion (86-90). One study found the maximal cycling workload to be slightly (4%) increased after eight weeks of coenzyme Q10 supplementation compared to placebo, although measures of aerobic power were not increased (91). Two studies actually found significantly greater improvement in measures of anaerobic (87) and aerobic (86) exercise performance with a placebo than with supplemental coenzyme Q10. More recent studies have suggested that coenzyme Q10 could help reduce both muscle damage-associated oxidative stress and low-grade inflammation induced by strenuous exercise (92-95). Studies on the effect of supplementation on physical performance in women are lacking, but there is little reason to suspect a gender difference in the response to coenzyme Q10 supplementation.
Sources
Biosynthesis
Coenzyme Q10 is synthesized in most human tissues. The biosynthesis of coenzyme Q10 involves three major steps: (1) synthesis of the benzoquinone structure from 4-hydroxybenzoate derived from either tyrosine or phenylalanine, two amino acids; (2) synthesis of the polyisoprenoid side chain from acetyl-coenzyme A (CoA) via the mevalonate pathway; and (3) the joining (condensation) of these two structures to form coenzyme Q10. In the mevalonate pathway, the enzyme 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, which converts HMG-CoA into mevalonate, is common to the biosynthetic pathways of both coenzyme Q10 and cholesterol and is inhibited by statins (cholesterol-lowering drugs; see Drug interactions) (1).
Of note, pantothenic acid (formerly vitamin B5) is the precursor of coenzyme A, and pyridoxine (vitamin B6), in the form of pyridoxal-5'-phosphate, is required for the conversion of tyrosine to 4-hydroxyphenylpyruvic acid that constitutes the first step in the biosynthesis of the benzoquinone structure of coenzyme Q10. It is not known to what extent the coenzyme Q10 biosynthetic pathway may be affected by inadequate pantothenic acid and/or vitamin B6 nutritional status.
Food sources
It has been estimated that dietary consumption contributes to about 25% of plasma coenzyme Q10, but there are currently no specific dietary intake recommendations for coenzyme Q10 from the US National Academy of Medicine (formerly the Institute of Medicine) or other agencies (96). The extent to which dietary consumption contributes to tissue coenzyme Q10 concentrations is not clear.
Based on studies employing food frequency questionnaires, the average dietary intake of coenzyme Q10 is about 3 to 6 mg/day (97). Rich sources of dietary coenzyme Q10 include mainly meat, poultry, and fish. Other good sources include soybean, corn, olive, and canola oils; nuts; and seeds. Fruit, vegetables, eggs, and dairy products are moderate sources of coenzyme Q10 (97). Some dietary sources are listed in Table 1.
| Food | Serving | Coenzyme Q10 (mg) |
|---|---|---|
| Beef, fried | 3 ounces* | 2.6 |
| Herring, marinated | 3 ounces | 2.3 |
| Chicken, fried | 3 ounces | 1.4 |
| Soybean oil | 1 tablespoon | 1.3 |
| Canola oil | 1 tablespoon | 1.0 |
| Rainbow trout, steamed | 3 ounces | 0.9 |
| Peanuts, roasted | 1 ounce | 0.8 |
| Sesame seeds, roasted | 1 ounce | 0.7 |
| Pistachio nuts, roasted | 1 ounce | 0.6 |
| Broccoli, boiled | ½ cup, chopped | 0.5 |
| Cauliflower, boiled | ½ cup, chopped | 0.4 |
| Orange | 1 medium | 0.3 |
| Strawberries | ½ cup | 0.1 |
| Egg, boiled | 1 medium | 0.1 |
| *A three-ounce serving of meat or fish is about the size of a deck of cards. | ||
Supplements
Coenzyme Q10 is available without a prescription as a dietary supplement in the US. Doses in supplements for adults range from 30 to 100 mg/day, which are considerably higher than typically estimated dietary coenzyme Q10 intakes. Coenzyme Q10 is fat-soluble and is best absorbed with fat in a meal. Doses higher than 100 mg/day are generally divided into two or three doses throughout the day (101). Less than 5% of orally administered coenzyme Q10 is thought to reach the circulation (102). Therefore, pharmacological doses of coenzyme Q10 as high as 1,200 to 3,000 mg/day for adults and 30 mg/kg/day for children are usually needed to relieve symptoms in patients with coenzyme Q10 deficiency (26).
Does oral coenzyme Q10 supplementation increase tissue concentrations?
Oral supplementation with coenzyme Q10 is known to increase blood and lipoprotein concentrations of coenzyme Q10 in humans (2, 15, 22). Plasma coenzyme Q10 appears to reach a plateau following supplementation with a dose of 2,400 mg/day (103, 104). Yet, under normal circumstances, uptake of supplemental coenzyme Q10 from peripheral tissues/organs is likely limited because coenzyme Q10 is ubiquitously synthesized (105). Nonetheless, under certain physiological circumstances (e.g., aging) or in pathologies, coenzyme Q10 status might be compromised and it is then presumed that supplementation might increase coenzyme Q10 concentrations in tissues that are deficient (106). For example, a study in 24 older adults supplemented with 300 mg/day of coenzyme Q10 or placebo for at least seven days prior to cardiac surgery found that the coenzyme Q10 content of atrial tissue was significantly increased in those taking coenzyme Q10, especially in patients greater than 70 years of age (38). In another study of patients with left ventricular dysfunction, supplementation with 150 mg/day of coenzyme Q10 for four weeks before cardiac surgery increased coenzyme Q10 concentrations in the heart but not in skeletal muscle (107). Finally, a 2007 review of the literature highlighted that plasma coenzyme Q10 concentrations higher than ‘normal’ were likely needed to promote coenzyme Q10 uptake by peripheral tissues and different tissues may indeed require different plasma thresholds for the uptake of coenzyme Q10 (102).
Safety
Toxicity
There have been no reports of significant adverse side effects of oral coenzyme Q10 supplementation at doses as high as 3,000 mg/day for up to eight months (103), 1,200 mg/day for up to 16 months (65), and 600 mg/day for up to 30 months (73). According to the observed safe level (OSL) risk assessment method, evidence of safety is strong with doses up to 1,200 mg/day of coenzyme Q10 (108). Some people have experienced gastrointestinal symptoms, such as nausea, diarrhea, appetite suppression, heartburn, and abdominal discomfort, especially with daily doses ≥200 mg (109). These adverse effects may be minimized if daily doses >100 mg are divided into two or three daily doses (101). During pregnancy, the use of coenzyme Q10 supplements (100 mg twice daily) from 20 weeks' gestation was found to be safe (110). Because reliable data in lactating women are not available, supplementation should be avoided during breast-feeding (110).
Drug interactions
Warfarin
Concomitant use of warfarin (Coumadin) and coenzyme Q10 supplements has been reported to decrease the anticoagulant effect of warfarin in a few cases (111). An individual on warfarin should not begin taking coenzyme Q10 supplements without consulting the health care provider who is managing his or her anticoagulant therapy. If warfarin and coenzyme Q10 are to be used concomitantly, blood tests to assess clotting time (prothrombin time; PT/INR) should be monitored frequently, especially in the first two weeks.
HMG-CoA reductase inhibitors (statins)
HMG-CoA reductase is an enzyme that catalyzes a biochemical reaction that is common to both cholesterol and coenzyme Q10 biosynthetic pathways (see Biosynthesis). Statins are HMG-CoA reductase inhibitors that are widely used as cholesterol-lowering medications. Statins can thus also reduce the endogenous synthesis of coenzyme Q10. Therapeutic use of statins, including simvastatin (Zocor), pravastatin (Pravachol), lovastatin (Mevacor, Altocor, Altoprev), rosuvastatin (Crestor), and atorvastatin (Lipitor), has been shown to decrease circulating coenzyme Q10 concentrations (112-121). However, because coenzyme Q10 circulates with lipoproteins, plasma coenzyme Q10 concentration is influenced by the concentration of circulating lipids (122, 123). It is likely that circulating coenzyme Q10 concentrations are decreased because statins reduce circulating lipids rather than because they inhibit coenzyme Q10 synthesis (124). In addition, very few studies have examined coenzyme Q10 concentrations in tissues other than blood such that the extent to which statin therapy affects coenzyme Q10 concentrations in the body's tissues is unknown (118, 120, 125). Finally, there is currently little evidence to suggest that secondary coenzyme Q10 deficiency is responsible for statin-associated muscle symptoms in treated patients. In addition, supplementation with coenzyme Q10 failed to relieve myalgia in statin-treated patients (see Disease Treatment) (126, 127).
Authors and Reviewers
Originally written in 2003 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in February 2007 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in March 2012 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in April 2018 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in May 2018 by:
Roland Stocker, Ph.D.
Centre for Vascular Research
School of Medical Sciences (Pathology) and
Bosch Institute
Sydney Medical School
The University of Sydney
Sydney, New South Wales, Australia
Copyright 2003-2024 Linus Pauling Institute
References
1. Acosta MJ, Vazquez Fonseca L, Desbats MA, et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta. 2016;1857(8):1079-1085. (PubMed)
2. Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591-598. (PubMed)
3. Nohl H, Gille L. The role of coenzyme Q in lysosomes. In: Kagan VEQ, P. J., ed. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; 2001:99-106.
4. Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7 Suppl:S34-40. (PubMed)
5. Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271(1):195-204. (PubMed)
6. Thomas SR, Stocker R. Mechanisms of antioxidant action of ubiquinol-10 for low-density lipoprotein. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; 2001:131-150.
7. Fazakerley DJ, Chaudhuri R, Yang P, et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife. 2018;7. (PubMed)
8. Kagan VE, Fabisak JP, Tyurina YY. Independent and concerted antioxidant functions of coenzyme Q. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; 2001:119-130.
9. Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53(10):764-770. (PubMed)
10. Hargreaves IP. Coenzyme Q10 as a therapy for mitochondrial disease. Int J Biochem Cell Biol. 2014;49:105-111. (PubMed)
11. Fragaki K, Chaussenot A, Benoist JF, et al. Coenzyme Q10 defects may be associated with a deficiency of Q10-independent mitochondrial respiratory chain complexes. Biol Res. 2016;49:4. (PubMed)
12. Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24(7):579-584. (PubMed)
13. Hernandez-Camacho JD, Bernier M, Lopez-Lluch G, Navas P. Coenzyme Q10 Supplementation in Aging and Disease. Front Physiol. 2018;9:44. (PubMed)
14. Beckman KB, Ames BN. Mitochondrial aging: open questions. Ann N Y Acad Sci. 1998;854:118-127. (PubMed)
15. Singh RB, Niaz MA, Kumar A, Sindberg CD, Moesgaard S, Littarru GP. Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors. 2005;25(1-4):219-224. (PubMed)
16. Sohal RS, Kamzalov S, Sumien N, et al. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic Biol Med. 2006;40(3):480-487. (PubMed)
17. Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/- mice. J Biol Chem. 2008;283(38):26217-26227. (PubMed)
18. Schmelzer C, Kubo H, Mori M, et al. Supplementation with the reduced form of coenzyme Q10 decelerates phenotypic characteristics of senescence and induces a peroxisome proliferator-activated receptor-alpha gene expression signature in SAMP1 mice. Mol Nutr Food Res. 2010;54(6):805-815. (PubMed)
19. Tian G, Sawashita J, Kubo H, et al. Ubiquinol-10 supplementation activates mitochondria functions to decelerate senescence in senescence-accelerated mice. Antioxid Redox Signal. 2014;20(16):2606-2620. (PubMed)
20. Johansson P, Dahlstrom O, Dahlstrom U, Alehagen U. Improved health-related quality of life, and more days out of hospital with supplementation with selenium and coenzyme Q10 combined. Results from a double-blind, placebo-controlled prospective study. J Nutr Health Aging. 2015;19(9):870-877. (PubMed)
21. Alehagen U, Aaseth J, Alexander J, Johansson P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS One. 2018;13(4):e0193120. (PubMed)
22. Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126(3):247-254. (PubMed)
23. Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic Biol Med. 2000;29(3-4):295-305. (PubMed)
24. Thomas SR, Leichtweis SB, Pettersson K, et al. Dietary cosupplementation with vitamin E and coenzyme Q(10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc Biol. 2001;21(4):585-593. (PubMed)
25. Turunen M, Wehlin L, Sjoberg M, et al. beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem Biophys Res Commun. 2002;296(2):255-260. (PubMed)
26. Rahman S, Clarke CF, Hirano M. 176th ENMC International Workshop: diagnosis and treatment of coenzyme Q(1)(0) deficiency. Neuromuscul Disord. 2012;22(1):76-86. (PubMed)
27. Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130(Pt 8):2037-2044. (PubMed)
28. Pineda M, Montero R, Aracil A, et al. Coenzyme Q(10)-responsive ataxia: 2-year-treatment follow-up. Mov Disord. 2010;25(9):1262-1268. (PubMed)
29. Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90(1):24-34. (PubMed)
30. Potgieter M, Pretorius E, Pepper MS. Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutr Rev. 2013;71(3):180-188. (PubMed)
31. Trupp RJ, Abraham WT. Congestive heart failure. In: Rakel RE, Bope ET, eds. Rakel: Conn's Current Therapy 2002. 54th ed. New York: W. B. Saunders Company; 2002:306-313.
32. McMurray JJ, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol. 2010;56(15):1196-1204. (PubMed)
33. Madmani ME, Yusuf Solaiman A, Tamr Agha K, et al. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev. 2014(6):Cd008684. (PubMed)
34. Lei L, Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC Cardiovasc Disord. 2017;17(1):196. (PubMed)
35. Pierce JD, Mahoney DE, Hiebert JB, et al. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or D-ribose. BMC Cardiovasc Disord. 2018;18(1):57. (PubMed)
36. Milei J, Forcada P, Fraga CG, et al. Relationship between oxidative stress, lipid peroxidation, and ultrastructural damage in patients with coronary artery disease undergoing cardioplegic arrest/reperfusion. Cardiovasc Res. 2007;73(4):710-719. (PubMed)
37. Liang S, Ping Z, Ge J. Coenzyme Q10 regulates antioxidative stress and autophagy in acute myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. 2017;2017:9863181. (PubMed)
38. Rosenfeldt FL, Pepe S, Linnane A, et al. The effects of ageing on the response to cardiac surgery: protective strategies for the ageing myocardium. Biogerontology. 2002;3(1-2):37-40. (PubMed)
39. Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. Biofactors. 1999;9(2-4):273-284. (PubMed)
40. Makhija N, Sendasgupta C, Kiran U, et al. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2008;22(6):832-839. (PubMed)
41. Taggart DP, Jenkins M, Hooper J, et al. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann Thorac Surg. 1996;61(3):829-833. (PubMed)
42. Leong JY, van der Merwe J, Pepe S, et al. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. 2010;19(10):584-591. (PubMed)
43. Celik T, Iyisoy A. Coenzyme Q10 and coronary artery bypass surgery: what we have learned from clinical trials. J Cardiothorac Vasc Anesth. 2009;23(6):935-936. (PubMed)
44. Huang CH, Kuo CL, Huang CS, et al. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine (Baltimore). 2016;95(31):e4501. (PubMed)
45. Aslanabadi N, Safaie N, Asgharzadeh Y, et al. The randomized clinical trial of coenzyme Q10 for the prevention of periprocedural myocardial injury following elective percutaneous coronary intervention. Cardiovasc Ther. 2016;34(4):254-260. (PubMed)
46. Tran MT, Mitchell TM, Kennedy DT, Giles JT. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy. 2001;21(7):797-806. (PubMed)
47. Ho MJ, Li EC, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2016;3:Cd007435. (PubMed)
48. Tabrizi R, Akbari M, Sharifi N, et al. The effects of coenzyme Q10 supplementation on blood pressures among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2018;25(1):41-50. (PubMed)
49. Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):311-316. (PubMed)
50. Fan L, Feng Y, Chen GC, Qin LQ, Fu CL, Chen LH. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2017;119:128-136. (PubMed)
51. Mazidi M, Kengne AP, Banach M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2018;128:130-136. (PubMed)
52. Zhai J, Bo Y, Lu Y, Liu C, Zhang L. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0170172. (PubMed)
53. Sahebkar A, Simental-Mendia LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: A systematic review and meta-analysis. Pharmacol Res. 2016;105:198-209. (PubMed)
54. Suksomboon N, Poolsup N, Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2015;40(4):413-418. (PubMed)
55. Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond). 2010;7:55. (PubMed)
56. McDonnell MG, Archbold GP. Plasma ubiquinol/cholesterol ratios in patients with hyperlipidaemia, those with diabetes mellitus and in patients requiring dialysis. Clin Chim Acta. 1996;253(1-2):117-126. (PubMed)
57. Lim SC, Tan HH, Goh SK, et al. Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q(10). Diabet Med. 2006;23(12):1344-1349. (PubMed)
58. Alcolado JC, Laji K, Gill-Randall R. Maternal transmission of diabetes. Diabet Med. 2002;19(2):89-98. (PubMed)
59. Suzuki S, Hinokio Y, Ohtomo M, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia. 1998;41(5):584-588. (PubMed)
60. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600-609. (PubMed)
61. Gotz ME, Gerstner A, Harth R, et al. Altered redox state of platelet coenzyme Q10 in Parkinson's disease. J Neural Transm. 2000;107(1):41-48. (PubMed)
62. Shults CW, Haas RH, Passov D, Beal MF. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol. 1997;42(2):261-264. (PubMed)
63. Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q-10 and 8-hydroxy-2'-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson's disease demonstrate that mitochondrial oxidative damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci Lett. 2010;469(1):159-163. (PubMed)
64. Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson's disease patients. Neurosci Lett. 2008;447(1):17-19. (PubMed)
65. Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541-1550. (PubMed)
66. Beal MF, Oakes D, Shoulson I, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71(5):543-552. (PubMed)
67. Yoritaka A, Kawajiri S, Yamamoto Y, et al. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord. 2015;21(8):911-916. (PubMed)
68. Negida A, Menshawy A, El Ashal G, et al. Coenzyme Q10 for patients with Parkinson's disease: a systematic review and meta-analysis. CNS Neurol Disord Drug Targets. 2016;15(1):45-53. (PubMed)
69. Zhu ZG, Sun MX, Zhang WL, Wang WW, Jin YM, Xie CL. The efficacy and safety of coenzyme Q10 in Parkinson's disease: a meta-analysis of randomized controlled trials. Neurol Sci. 2017;38(2):215-224. (PubMed)
70. Ferrante RJ, Andreassen OA, Dedeoglu A, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington's disease. J Neurosci. 2002;22(5):1592-1599. (PubMed)
71. Stack EC, Smith KM, Ryu H, et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington's disease mice. Biochim Biophys Acta. 2006;1762(3):373-380. (PubMed)
72. Yang L, Calingasan NY, Wille EJ, et al. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J Neurochem. 2009;109(5):1427-1439. (PubMed)
73. The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 2001;57(3):397-404. (PubMed)
74. Hyson HC, Kieburtz K, Shoulson I, et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. Mov Disord. 2010;25(12):1924-1928. (PubMed)
75. McGarry A, McDermott M, Kieburtz K, et al. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88(2):152-159. (PubMed)
76. Burk K. Friedreich Ataxia: current status and future prospects. Cerebellum Ataxias. 2017;4:4. (PubMed)
77. Strawser C, Schadt K, Hauser L, et al. Pharmacological therapeutics in Friedreich ataxia: the present state. Expert Rev Neurother. 2017;17(9):895-907. (PubMed)
78. Lodi R, Hart PE, Rajagopalan B, et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Ann Neurol. 2001;49(5):590-596. (PubMed)
79. Hart PE, Lodi R, Rajagopalan B, et al. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol. 2005;62(4):621-626. (PubMed)
80. Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. 2008;15(12):1371-1379. (PubMed)
81. Lo RY, Figueroa KP, Pulst SM, et al. Coenzyme Q10 and spinocerebellar ataxias. Mov Disord. 2015;30(2):214-220. (PubMed)
82. Cornelius N, Wardman JH, Hargreaves IP, et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fibroblasts: Effect of coenzyme Q10 supplementation on these parameters. Mitochondrion. 2017;34:103-114. (PubMed)
83. Folkers K, Osterborg A, Nylander M, Morita M, Mellstedt H. Activities of vitamin Q10 in animal models and a serious deficiency in patients with cancer. Biochem Biophys Res Commun. 1997;234(2):296-299. (PubMed)
84. Lesser GJ, Case D, Stark N, et al. A randomized, double-blind, placebo-controlled study of oral coenzyme Q10 to relieve self-reported treatment-related fatigue in newly diagnosed patients with breast cancer. J Support Oncol. 2013;11(1):31-42. (PubMed)
85. Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support Care Cancer. 2016;24(2):637-646. (PubMed)
86. Laaksonen R, Fogelholm M, Himberg JJ, Laakso J, Salorinne Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol. 1995;72(1-2):95-100. (PubMed)
87. Malm C, Svensson M, Ekblom B, Sjodin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand. 1997;161(3):379-384. (PubMed)
88. Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr. 1997;7(3):197-206. (PubMed)
89. Porter DA, Costill DL, Zachwieja JJ, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med. 1995;16(7):421-427. (PubMed)
90. Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Int J Sport Nutr. 1991;1(4):353-365. (PubMed)
91. Bonetti A, Solito F, Carmosino G, Bargossi AM, Fiorella PL. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J Sports Med Phys Fitness. 2000;40(1):51-57. (PubMed)
92. Abdizadeh L, Jafari A, Armanfar M. Effects of short-term coenzyme Q10 supplementation on markers of oxidative stress and inflammation after downhill running in male mountaineers. Science & Sports. 2015;30(6):328-334.
93. Díaz-Castro J, Guisado R, Kajarabille N, et al. Coenzyme Q(10) supplementation ameliorates inflammatory signaling and oxidative stress associated with strenuous exercise. Eur J Nutr. 2012;51(7):791-799. (PubMed)
94. Leelarungrayub D, Rawattikanon A, Klaphajone J, Pothong-sunan P, Bloomer RJ. Coenzyme Q10 supplementation decreases oxidative stress and improves physical performance in young swimmers Open Sports Med J 2010;4(1):1-8.
95. Ostman B, Sjodin A, Michaelsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Nutrition. 2012;28(4):403-417. (PubMed)
96. Weber C. Dietary intake and absorption of coenzyme Q. In: Kagan VE, Quinn PJ, eds. Coenzyme Q: Molecular Mechanisms in Health and Disease. Boca Raton: CRC Press; 2001:209-215.
97. Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50(4):269-280. (PubMed)
98. Mattila P, Kumpulainen J. Coenzymes Q9 and Q10: Contents in foods and dietary intake. J Food Comp Anal. 2001;14(4):409-417.
99. Kamei M, Fujita T, Kanbe T, et al. The distribution and content of ubiquinone in foods. Int J Vitam Nutr Res. 1986;56(1):57-63. (PubMed)
100. Weber C, Bysted A, Holmer G. Coenzyme Q10 in the diet--daily intake and relative bioavailability. Mol Aspects Med. 1997;18 Suppl:S251-254. (PubMed)
101. Natural Medicines. Coenzyme Q10. Professional handout/Adverse effects. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 4/23/18.
102. Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7 Suppl:S78-88. (PubMed)
103. Ferrante KL, Shefner J, Zhang H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65(11):1834-1836. (PubMed)
104. Shults CW, Flint Beal M, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Exp Neurol. 2004;188(2):491-494. (PubMed)
105. Svensson M, Malm C, Tonkonogi M, Ekblom B, Sjodin B, Sahlin K. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Int J Sport Nutr. 1999;9(2):166-180. (PubMed)
106. Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40(5):445-453. (PubMed)
107. Keith M, Mazer CD, Mikhail P, Jeejeebhoy F, Briet F, Errett L. Coenzyme Q10 in patients undergoing CABG: Effect of statins and nutritional supplementation. Nutr Metab Cardiovasc Dis. 2008;18(2):105-111. (PubMed)
108. Hathcock JN, Shao A. Risk assessment for coenzyme Q10 (Ubiquinone). Regul Toxicol Pharmacol. 2006;45(3):282-288. (PubMed)
109. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. Montvale: Thomson Reuters; 2008.
110. Natural Medicines. Coenzyme Q10. Professional handout/Safety. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 4/23/18.
111. Natural Medicines. Coenzyme Q10. Professional handout/Drug Interactions. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 4/23/18.
112. Folkers K, Langsjoen P, Willis R, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. 1990;87(22):8931-8934. (PubMed)
113. Colquhoun DM, Jackson R, Walters M, et al. Effects of simvastatin on blood lipids, vitamin E, coenzyme Q10 levels and left ventricular function in humans. Eur J Clin Invest. 2005;35(4):251-258. (PubMed)
114. Mabuchi H, Higashikata T, Kawashiri M, et al. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb. 2005;12(2):111-119. (PubMed)
115. Bargossi AM, Battino M, Gaddi A, et al. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Int J Clin Lab Res. 1994;24(3):171-176. (PubMed)
116. Watts GF, Castelluccio C, Rice-Evans C, Taub NA, Baum H, Quinn PJ. Plasma coenzyme Q (ubiquinone) concentrations in patients treated with simvastatin. J Clin Pathol. 1993;46(11):1055-1057. (PubMed)
117. Ghirlanda G, Oradei A, Manto A, et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33(3):226-229. (PubMed)
118. Laaksonen R, Jokelainen K, Laakso J, et al. The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol. 1996;77(10):851-854. (PubMed)
119. Laaksonen R, Ojala JP, Tikkanen MJ, Himberg JJ. Serum ubiquinone concentrations after short- and long-term treatment with HMG-CoA reductase inhibitors. Eur J Clin Pharmacol. 1994;46(4):313-317. (PubMed)
120. Elmberger PG, Kalen A, Lund E, et al. Effects of pravastatin and cholestyramine on products of the mevalonate pathway in familial hypercholesterolemia. J Lipid Res. 1991;32(6):935-940. (PubMed)
121. Ashton E, Windebank E, Skiba M, et al. Why did high-dose rosuvastatin not improve cardiac remodeling in chronic heart failure? Mechanistic insights from the UNIVERSE study. Int J Cardiol. 2011;146(3):404-407. (PubMed)
122. Hughes K, Lee BL, Feng X, Lee J, Ong CN. Coenzyme Q10 and differences in coronary heart disease risk in Asian Indians and Chinese. Free Radic Biol Med. 2002;32(2):132-138. (PubMed)
123. Hargreaves IP, Duncan AJ, Heales SJ, Land JM. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: possible biochemical/clinical implications. Drug Saf. 2005;28(8):659-676. (PubMed)
124. Stocker R, Pollicino C, Gay CA, et al. Neither plasma coenzyme Q10 concentration, nor its decline during pravastatin therapy, is linked to recurrent cardiovascular disease events: a prospective case-control study from the LIPID study. Atherosclerosis. 2006;187(1):198-204. (PubMed)
125. Laaksonen R, Jokelainen K, Sahi T, Tikkanen MJ, Himberg JJ. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther. 1995;57(1):62-66. (PubMed)
126. Tan JT, Barry AR. Coenzyme Q10 supplementation in the management of statin-associated myalgia. Am J Health Syst Pharm. 2017;74(11):786-793. (PubMed)
127. Taylor BA. Does coenzyme Q10 supplementation mitigate statin-associated muscle symptoms? Pharmacological and methodological considerations. Am J Cardiovasc Drugs. 2018;18(2):75-82. (PubMed)