Contents
Iron is the fourth most abundant element of Earth’s crust and one of the best studied micronutrients in nutrition science (1, 2). It is a key element in the metabolism of all living organisms. Iron exists in two biologically relevant oxidation states: the ferrous form (Fe2+) and the ferric form (Fe3+). Iron is an essential component of hundreds of proteins and enzymes supporting essential biological functions, such as oxygen transport, energy production, DNA synthesis, and cell growth and replication.
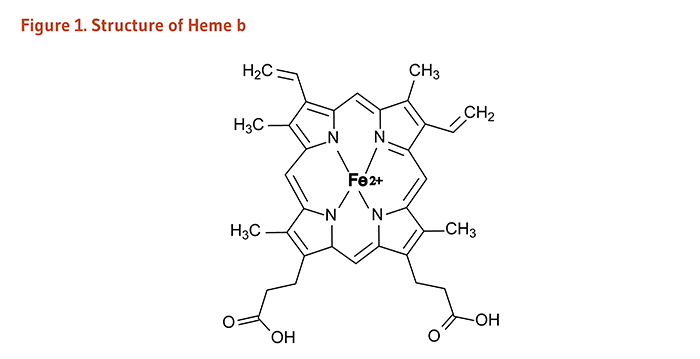
Heme is an iron-containing compound found in a number of biologically important molecules (Figure 1). Some, but not all, iron-dependent proteins are heme-containing proteins (also called hemoproteins). Iron-dependent proteins that carry out a broad range of biological activities may be classified as follows (1, 3):
Iron-containing proteins support a number of functions, some of which are listed below.
Globin-hemes are heme-containing proteins that are involved in the transport and storage of oxygen and, to a lesser extent, may act as free radical scavengers (1). Hemoglobin is the primary protein found in red blood cells and represents about two-thirds of the body's iron (3). The vital role of hemoglobin in transporting oxygen from the lungs to the rest of the body is derived from its unique ability to acquire oxygen rapidly during the short time it spends in contact with the lungs and to release oxygen as needed during its circulation through the tissues. Myoglobin functions in the transport and short-term storage of oxygen in muscle cells, helping to match the supply of oxygen to the demand of working muscles (1). A third globin called neuroglobin is preferentially expressed in the central nervous system, but its function is not well understood (4).
Cytochromes are heme-containing enzymes that have important roles in mitochondrial electron transport required for cellular energy production and thus life. Specifically, cytochromes serve as electron carriers during the synthesis of ATP, the primary energy storage compound in cells. Cytochrome P450 (CYP) is a family of enzymes involved in the metabolism of a number of important biological molecules (including organic acids; fatty acids; prostaglandins; steroids; sterols; and vitamins A, D, and K), as well as in the detoxification and metabolism of drugs and pollutants. Nonheme iron-containing enzymes in the citric acid cycle, such as NADH dehydrogenase and succinate dehydrogenase, are also critical to energy metabolism (1).
Catalase and some peroxidases are heme-containing enzymes that protect cells against the accumulation of hydrogen peroxide, a potentially damaging reactive oxygen species (ROS), by catalyzing a reaction that converts hydrogen peroxide to water and oxygen. As part of the immune response, some white blood cells engulf bacteria and expose them to ROS in order to kill them. The synthesis of one such ROS, hypochlorous acid, by neutrophils is catalyzed by the heme-containing enzyme myeloperoxidase (1).
In addition, in the thyroid gland, heme-containing thyroid peroxidase catalyzes the iodination of thyroglobulin for the production of thyroid hormones such that thyroid metabolism can be impaired in iron deficiency and iron-deficiency anemia (see Nutrient Interactions).
Inadequate oxygen (hypoxia), such as that experienced by those who live at high altitudes or those with chronic lung disease, induces compensatory physiologic responses, including increased red blood cell formation (erythropoiesis), increased blood vessel growth (angiogenesis), and increased production of enzymes utilized in anaerobic metabolism. Hypoxia is also observed in pathological conditions like ischemia/stroke and inflammatory disorders. Under hypoxic conditions, transcription factors known as hypoxia-inducible factors (HIF) bind to response elements in genes that encode various proteins involved in compensatory responses to hypoxia and increase their synthesis. Iron-dependent enzymes of the dioxygenase family, HIF prolyl hydroxylases and asparaginyl hydroxylase (factor inhibiting HIF-1 [FIH-1]), have been implicated in HIF regulation. When cellular oxygen tension is adequate, newly synthesized HIF-α subunits (HIF-1α, HIF-2α, HIF-3α) are modified by HIF prolyl hydroxylases in an iron/2-oxoglutarate-dependent process that targets HIF-α for rapid degradation. FIH-1-induced asparaginyl hydroxylation of HIF-α impairs the recruitment of co-activators to HIF-α transcriptional complex and therefore prevents HIF-α transcriptional activity. When cellular oxygen tension drops below a critical threshold, prolyl hydroxylase can no longer target HIF-α for degradation, allowing HIF-α to bind to HIF-1β and form a transcription complex that enters the nucleus and binds to specific hypoxia response elements (HRE) on target genes like the erythropoietin gene (EPO) (5).
Ribonucleotide reductases (RNRs) are iron-dependent enzymes that catalyze the synthesis of deoxyribonucleotides required for DNA replication. RNRs also facilitate DNA repair in response to DNA damage. Other enzymes essential for DNA synthesis and repair, such as DNA polymerases and DNA helicases, are Fe-S cluster proteins. Although the underlying mechanisms are still unclear, depletion of intracellular iron was found to inhibit cell cycle progression, growth, and division. Inhibition of heme synthesis also induced cell cycle arrest in breast cancer cells (6).
Iron is required for a number of additional vital functions, including growth, reproduction, healing, and immune function.
While iron is an essential mineral, it is potentially toxic because free iron inside the cell can lead to the generation of free radicals causing oxidative stress and cellular damage. Thus, it is important for the body to systemically regulate iron homeostasis. The body tightly regulates the transport of iron throughout various body compartments, such as developing red blood cells (erythroblasts), circulating macrophages, liver cells (hepatocytes) that store iron, and other tissues (7). Intracellular iron concentrations are regulated according to the body’s iron needs (see below), but extracellular signals also regulate iron homeostasis in the body through the action of hepcidin.
Hepcidin, a peptide hormone primarily synthesized by liver cells, is the key regulator of systemic iron homeostasis. Hepcidin can induce the internalization and degradation of the iron-efflux protein, ferroportin-1; ferroportin-1 regulates the release of iron from certain cells, such as enterocytes, hepatocytes, and iron-recycling macrophages, into plasma (8). When body iron concentration is low and in situations of iron-deficiency anemia, hepcidin expression is minimal, allowing for iron absorption from the diet and iron mobilization from body stores. In contrast, when there are sufficient iron stores or in the case of iron overload, hepcidin inhibits dietary iron absorption, promotes cellular iron sequestration, and reduces iron bioavailability. Hepcidin expression is up-regulated in conditions of inflammation and endoplasmic reticulum stress and down-regulated in hypoxia (9). In Type 2B hemochromatosis, deficiency in hepcidin due to mutations in the hepcidin gene, HAMP, causes abnormal iron accumulation in tissues (see Iron Overload). Of note, hepcidin is also thought to have a major antimicrobial role in the innate immune response by limiting iron availability to invading microorganisms (see Iron withholding defense during infection) (10).
Iron-responsive elements (IREs) are short sequences of nucleotides found in the messenger RNAs (mRNAs) that code for key proteins in the regulation of iron storage, transport, and utilization. Iron regulatory proteins (IRPs: IRP-1, IRP-2) can bind to IREs and control mRNA stability and translation, thereby regulating the synthesis of specific proteins, such as ferritin (iron storage protein) and transferrin receptor-1 (TfR; controls cellular iron uptake) (1, 2).
When the iron supply is low, iron is not available for storage or release into plasma. Less iron binds to IRPs, allowing the binding of IRPs to IREs. The binding of IRPs to IREs located in the 5’end of mRNAs coding for ferritin and ferroportin-1 (iron efflux protein) inhibits mRNA translation and protein synthesis. Translation of mRNA that codes for the key regulatory enzyme of heme synthesis in immature red blood cells is also reduced to conserve iron. In contrast, IRP binding to IREs in the 3’ end of mRNAs that code for TfR and divalent metal transporter-1 (DMT1) stimulates the synthesis of iron transporters, thereby increasing iron uptake into cells (1, 2).
When the iron supply is high, more iron binds to IRPs, thereby preventing the binding of IRPs to IREs on mRNAs. This allows for an increased synthesis of proteins involved in iron storage (ferritin) and efflux (ferroportin-1) and a decreased synthesis of iron transporters (TfR and DMT1) such that iron uptake is limited (2). In the brain, IRPs are also prevented from binding to the 5’end of amyloid precursor protein (APP) mRNA, allowing for APP expression. APP stimulates iron efflux from neurons through stabilizing ferroportin-1. In Parkinson’s disease (PD), APP expression is inappropriately suppressed, leading to iron accumulation in dopaminergic neurons (11, 12).
Iron is required by most infectious agents to grow and spread, as well as by the infected host in order to mount an effective immune response. Sufficient iron is critical for the differentiation and proliferation of T lymphocytes and the generation of reactive oxygen species (ROS) required for killing pathogens (13). During infection and inflammation, hepcidin synthesis is up-regulated, serum iron concentrations decrease, and concentrations of ferritin (the iron storage protein) increase, supporting the idea that sequestering iron from pathogens is an important host defense mechanism (2).
Total body content of iron in adults is estimated to be 2.3 g in women and 3.8 g in men (2). The body excretes very little iron; basal losses, menstrual blood loss, and the need of iron for the synthesis of new tissue are compensated by the daily absorption of a small proportion of dietary iron (1 to 2 mg/day). Body iron is primarily found in red blood cells, which contain 3.5 mg of iron per g of hemoglobin. Senescent red blood cells are engulfed by macrophages in the spleen, and about 20 mg of iron can be recovered daily from heme recycling. The released iron is either deposited to the ferritin of spleen macrophages or exported by ferroportin-1 (iron efflux protein) to transferrin (the main iron carrier in blood) that delivers iron to other tissues. Iron recycling is very efficient, with about 35 mg being recycled daily (1).
Measurements of iron stores, circulating iron, and hematological parameters may be used to assess the iron status of healthy people in the absence of inflammatory disorders, parasitic infection, and obesity. Commonly used iron status biomarkers include serum ferritin (iron-storage protein), serum iron, total iron binding capacity (TIBC), and saturation of transferrin (the main iron carrier in blood; TSAT). Soluble transferrin receptor (sTfR) is also an indicator of iron status when iron stores are depleted. In iron deficiency and iron-deficiency anemia, the abundance of cell surface-bound transferrin receptors that bind diferric transferrin is increased in order to maximize the uptake of available iron. Therefore, the concentration of sTfR generated by the cleavage of cell-bound transferrin receptors is increased in iron deficiency. Hematological markers, including hemoglobin concentration, mean corpuscular hemoglobin concentration, mean corpuscular volume of red blood cells, and reticulocyte hemoglobin content can help detect abnormality if anemia is present (9, 14).
Of note, serum ferritin is an acute-phase reactant protein that is up-regulated by inflammation. Importantly, serum hepcidin concentration is also increased by inflammation to limit iron availability to pathogens. Therefore, it is important to include inflammation markers (e.g., C-reactive protein, fibrinogen) when assessing iron status to rule out inflammation (14).
Vitamin A deficiency often coexists with iron deficiency and may exacerbate iron-deficiency anemia by altering iron metabolism (15). Vitamin A supplementation has been shown to have beneficial effects on iron-deficiency anemia and improve iron nutritional status among children and pregnant women (15, 16). The combination of vitamin A and iron seems to reduce anemia more effectively than either supplemental iron or vitamin A alone (17). Vitamin A may facilitate the mobilization of iron from storage sites to developing red blood cells for incorporation into hemoglobin (15, 16). Moreover, studies in rats have shown that iron deficiency alters plasma and liver levels of vitamin A (18, 19).
Adequate copper nutritional status is necessary for normal iron metabolism and red blood cell formation. Anemia is a clinical sign of copper deficiency, and iron has been found to accumulate in the livers of copper-deficient animals, indicating that copper (via copper-containing ceruloplasmin) is required for iron transport to the bone marrow for red blood cell formation (20). The connection between copper availability and iron metabolism has also been established in humans; copper deficiency can lead to secondary ceruloplasmin deficiency and hepatic iron overload and/or cirrhosis (21). Oral copper supplementation restored normal ceruloplasmin levels and plasma ferroxidase activity and corrected the iron metabolism disorder in a copper-deficient subject (22). Moreover, infants fed a high-iron formula absorbed less copper than infants fed a low-iron formula, suggesting that high iron intakes may interfere with copper absorption in infants (23).
Zinc is essential to maintain adequate erythropoiesis. When zinc deficiency coexists with iron deficiency, it may exacerbate iron-deficiency anemia (24). On the other hand, high doses of iron supplements, taken together with zinc supplements on an empty stomach, may inhibit the absorption of zinc. When taken with food, supplemental iron does not appear to inhibit zinc absorption. Iron-fortified foods have not been found to impair zinc absorption (25, 26).
The presence of calcium decreases iron absorption from both nonheme (i.e., most supplements and food sources other than meat, poultry, and seafood) and heme sources (27). However, calcium supplementation up to 12 weeks has not been found to change iron nutritional status, probably due to a compensatory increase in iron absorption (28). Individuals taking iron supplements should take them two hours apart from calcium-rich food or supplements to maximize iron absorption.
Severe iron-deficiency anemia can impair thyroid metabolism in the following ways: (1) by altering the thyroid-stimulating hormone response of the pituitary gland; (2) by reducing the activity of thyroid peroxidase that catalyzes the iodination of thyroglobulin for the production of thyroid hormones; and (3) in the liver by limiting the conversion of T4 to T3, increasing T3 turnover, and decreasing T3 binding to nuclear receptors (29). It is estimated that goiter and iron-deficiency anemia coexist in up to 25% of school-age children in west and north Africa (30). A randomized controlled study in iron-deficient children with goiter showed a greater reduction in thyroid size following the consumption of iodized salt together with 60 mg/day of iron four times per week compared to placebo (31). Additional interventions have confirmed that correcting iron-deficiency anemia improved the efficacy of iodine supplementation to mitigate thyroid disorders (reviewed in 29, 30).
Iron deficiency is the most common nutrient deficiency in the US and the world. Levels of iron deficiency are listed below from least to most severe.
Iron stores are depleted, but the functional iron supply is not limited.
Before the development of frank anemia, the supply of functional iron to tissues, including bone marrow, is inadequate such as to impair erythropoiesis.
By definition, anemia is present when individual hemoglobin concentrations fall below two standard deviations of the distribution mean for hemoglobin in a healthy population of the same gender and age and living at the same altitude (32). In 2013, iron-deficiency anemia was the leading cause of years lived with disability in children and adolescents in the 50 most populous countries. The countries with the highest prevalence of iron-deficiency anemia in individuals younger than 19 years were Afghanistan (41%) and Yemen (39.8%); India contributed the largest number of cases of anemia (147.9 million). The prevalence in the US was estimated to be 19.3% with nearly 16 million cases of iron-deficiency anemia in children and adolescents (33).
Iron-deficiency anemia occurs when there is inadequate iron to support normal red blood cell formation. The anemia of iron deficiency is usually characterized as microcytic and hypochromic, i.e., red blood cells are measurably smaller than normal and their hemoglobin content is decreased such that they are paler than normal. At this stage of iron deficiency, symptoms may be a result of inadequate oxygen delivery due to anemia and/or suboptimal function of iron-dependent enzymes. Changes in hematological parameters are used in the clinical diagnosis of iron-deficiency anemia (see Assessment of iron status). It is important to remember that iron deficiency is not the only cause of anemia, and that the diagnosis or treatment of iron deficiency solely on the basis of anemia may lead to misdiagnosis or inappropriate treatment of the underlying cause (34). See also the articles on Folate and Vitamin B12 for information on other nutritional causes of anemia.
Most of the symptoms of iron deficiency are a result of the associated anemia and may include fatigue, rapid heart rate, palpitations, and rapid breathing on exertion. Iron deficiency impairs athletic performance and physical work capacity in several ways. In iron-deficiency anemia, the reduced hemoglobin content of red blood cells results in decreased oxygen delivery to active tissues. Decreased myoglobin levels in muscle cells limit the amount of oxygen that can be delivered to mitochondria for oxidative metabolism. Iron depletion also decreases the oxidative capacity of muscle by diminishing the mitochondrial content of cytochromes and other iron-dependent enzymes required for electron transport and ATP synthesis (see Function) (35).
Poor thyroid function and impaired thyroid hormone synthesis likely disrupt the ability to maintain a normal body temperature on exposure to cold in iron-deficient individuals (see Function). Iron deficiency may also impair neutrophil phagocytosis and microbicidal activity and T-lymphocyte proliferative responses to infection (1). Severe iron-deficiency anemia may result in brittle and spoon-shaped nails, sores at the corners of the mouth, taste bud atrophy, and a sore tongue. In rare cases, advanced iron-deficiency anemia may cause difficulty in swallowing due to the formation of webs of tissue in the throat and esophagus due to a degradation of the pharyngeal muscles (36). The development of esophageal webs, also known as Plummer-Vinson syndrome, may require a genetic predisposition in addition to iron deficiency. Iron deficiency and iron-deficiency anemia in early childhood have been shown to impair psychomotor development and induce short- and long-term behavioral and cognitive alterations (reviewed in 37). Further, pica, a behavioral disturbance characterized by the consumption of non-food items, may be a symptom and a cause of iron deficiency (38).
Neonates and infants up to six months of age: Inadequate maternal iron body stores and anemia during pregnancy may reduce the duration of gestation and birth weight; preterm and/or low body-weight newborns are at increased risk of iron-deficiency anemia (14). Pregnancy complications, including preeclampsia and gestational diabetes mellitus, may also lead to low iron stores in preterm and term infants (14).
Most of the 150 to 250 mg of iron present in a full-term healthy newborn is accumulated during the third trimester of pregnancy and is sufficient for the first four to six months of life (34). Iron stores are essential for infants less than six months of age because breast milk is relatively poor in iron (0.2 mg/L-0.4 mg/L), and intestinal absorption of iron remains low until six months of age. High iron requirements during this period of sustained and rapid growth rate can worsen the deficit in body iron in preterm infants (14). Moreover, a review of randomized controlled trials suggested that infants with an early umbilical cord clamping (≤1 min after birth) are at least twice more likely to be iron-deficient at three to six months compared to those with delayed cord clamping (39). Yet, healthy full-term infants have little need for external sources of iron before six months of age (1).
Infants and children between the ages of 6 months and 3 years: A full-term infant's iron stores are usually sufficient to last for the early months of life, but there is an increased risk of iron deficiency for infants older than six months (1). Given the sustained need of iron for increasing tissue mass, blood volume, and replenishing iron stores, the recommended dietary allowance (RDA) for iron is 11 mg/day for infants aged seven to 12 months, as established by the US Institute of Medicine (see Table 1).
The RDA for iron is 7 mg/day for toddlers aged 1 to 3 years old. Based on the US National Health and Nutrition Examination Survey (NHANES) 1999-2002 data, the prevalence of iron deficiency in toddlers aged 12 to 35 months varies from 6.6%-15.2%, and the prevalence of iron-deficiency anemia is 0.9%-4.4%, depending on ethnicity and socioeconomic status (14).
Of note, the World Health Organization (WHO) and the American Academy of Pediatrics recommend universal screening for anemia at one year of age. Yet, a recent report by the US Preventive Services Task Force (USPSTF) stated there was insufficient evidence to assess the benefits versus harms of screening (34, 40).
Adolescents: Early adolescence is period of rapid growth. Blood loss that occurs with menstruation in adolescent girls adds to the increased iron requirement of adolescence (1). The RDA of iron is 11 mg/day and 15 mg/day for adolescent boys and girls, respectively (see Table 1).
Nonpregnant women of childbearing age: Based on data from NHANES 2003-2006, the percentage of US women with two out of three markers of iron status (i.e., hemoglobin, ferritin, and % transferrin saturation) below cutoff values for deficiency was 9.8% in nonpregnant women (41).
The use of oral contraceptives decreases menstrual blood losses and is thus associated with improved iron status compared to intrauterine devices (copper coil) (1).
Breast-feeding is associated with lower dietary iron needs, allowing for the repletion of iron stores depleted during pregnancy and delivery. However, iron repletion may be incomplete in high-parity women who are therefore at increased risk of iron deficiency (41).
Pregnant women: The requirement for iron is significantly increased during pregnancy due to increased iron utilization by the developing fetus and placenta, as well as maternal blood volume expansion (42). Analysis of data from NHANES 2005-2006 found that 18.1% of pregnant women (mean age, 27.5 years) were deficient in iron, as assessed by the log ratio of soluble transferrin receptor to serum ferritin (43). The prevalence of iron deficiency was greater during the second (20.7%) and third (29.7%) trimesters compared to the first trimester (4.5%) of gestation. Further, iron deficiency in pregnancy was found to be more prevalent in Mexican (23.6%) and Black Americans (29.6%) than in non-Hispanic White Americans (13.9%) (43).
Chronic bleeding or acute blood loss may result in iron deficiency. One milliliter (mL) of blood with a hemoglobin concentration of 150 g/L contains 0.5 mg of iron. Thus, chronic loss of very small amounts of blood may result in iron deficiency.
Parasitic infestation: A common cause of chronic blood loss and iron deficiency in developing countries is intestinal parasitic infection (44).
Frequent blood donation: Individuals who donate blood frequently, especially menstruating women, may need to increase their iron intake to prevent deficiency because each 500 mL of blood donated contains between 200 and 250 mg of iron (45, 46).
Regular intense exercise: Daily iron losses have been found to be greater in athletes involved in intense endurance training. This may be due to expanding blood cell mass and muscle mass, increased microscopic bleeding from the gastrointestinal tract (with the regular use of anti-inflammatory drugs), or increased fragility and hemolysis of red blood cells (47). The Food and Nutrition Board estimates that the average requirement for iron may be 30% higher for those who engage in regular intense exercise (25).
Celiac disease: Celiac disease (celiac sprue) is an autoimmune disorder estimated to occur in 1% of the population. When people with celiac disease consume food or products that contain gluten, the immune system response damages the intestinal mucosa, which may result in nutrient malabsorption and iron-deficiency anemia (48).
Atrophic gastritis: This condition is usually associated with the presence of antibodies directed towards stomach cells and has been implicated in pernicious anemia (see the article on Vitamin B12). Atrophic gastritis simultaneously impairs the absorption of both vitamin B12 and iron; yet, in menstruating women, iron deficiency may occur years before the depletion of vitamin B12 body stores (47).
Helicobacter pylori infection: H. pylori infection is associated with iron-deficiency anemia, especially in children, even in the absence of gastrointestinal bleeding. Data from NHANES 2000-2001 in individuals older than three years showed that the presence of iron deficiency (based on serum ferritin concentrations) was 40% more prevalent in those infected with H. pylori than in H. pylori-free individuals (49). Occult gastrointestinal bleeding and competition for dietary iron by bacteria may explain iron deficiency in infected individuals. Moreover, Helicobacter pylori infection may also play a role in the pathogenesis of atrophic gastritis (47).
Inflammatory bowel diseases (IBD): Iron-deficiency anemia is commonly reported among patients with IBD (e.g., ulcerative colitis, Crohn’s disease), likely due to both impaired intestinal absorption of iron and blood loss from ulcerated mucosa (50).
Gastric bypass surgery: Some types of gastric bypass (bariatric) surgery increase the risk of iron deficiency by causing malabsorption of iron, among other nutrients (51).
Obesity: An inverse association between body weight and iron status has been reported in several observational studies in children and adults (52, 53). Higher hepcidin expression in obese people may impair iron absorption despite adequate dietary intake of iron. Weight loss might lower serum hepcidin concentration and improve iron status in obese individuals (9).
Anemia of chronic disease: Acute and chronic inflammation may lead to abnormally low circulating concentrations of iron and to the development of anemia. This type of anemia of inflammation, also known as anemia of chronic disease (ACD), is commonly observed in inflammatory disorders, cancer, critical illness, trauma, chronic infection, and parasitic infestation. It is thought that anemia develops because dietary iron absorption and iron mobilization from body stores are inhibited by inflammation-induced hepcidin up-regulation (see also Systemic regulation of iron homeostasis) (9).
Vegetarian diet with inadequate sources of iron: Because iron from plants (nonheme iron) is less efficiently absorbed than that from animal sources (see Sources), the US Food and Nutrition Board (FNB) of the Institute of Medicine (IOM) estimated that the bioavailability of iron from a vegetarian diet was only 10% versus 18% from a mixed Western diet. Therefore, the recommended dietary allowance (RDA) of iron for individuals consuming a completely vegetarian diet may be 1.8 times higher than the RDA for non-vegetarians (25). Yet, a vegetarian diet does not appear to be associated with an increased risk of iron deficiency when it includes whole grains, legumes, nuts, seeds, dried fruit, iron-fortified cereal, and green leafy vegetables (see Sources) (54).
Chronic kidney disease (CKD): Iron losses in CKD patients are due to significant gastrointestinal blood loss (1.2 L blood loss/year corresponding to ~400 mg iron/year) compared to individuals with normal kidney function (0.83 mL blood loss/day corresponding to ~100 mg iron/year). Estimated blood losses are even larger in patients on hemodialysis, and iron losses may be 1,000 to 2,000 mg/year or higher. Persistent inflammation in CKD patients may also contribute to inadequate iron supply for red blood cell formation despite adequate body iron stores (55).
The RDA for iron was revised in 2001 and is based on the prevention of iron deficiency and maintenance of adequate iron stores in individuals eating a mixed diet (Table 1; 25).
| Life Stage | Age | Males (mg/day) | Females (mg/day) |
|---|---|---|---|
| Infants | 0-6 months | 0.27 (AI) | 0.27 (AI) |
| Infants | 7-12 months | 11 | 11 |
| Children | 1-3 years | 7 | 7 |
| Children | 4-8 years | 10 | 10 |
| Children | 9-13 years | 8 | 8 |
| Adolescents | 14-18 years | 11 | 15 |
| Adults | 19-50 years | 8 | 18 |
| Adults | 51 years and older | 8 | 8 |
| Pregnancy | all ages | - | 27 |
| Breast-feeding | 18 years and younger | - | 10 |
| Breast-feeding | 19 years and older | - | 9 |
Prevention or alleviation of iron deficiency or iron-deficiency anemia can limit the impact of iron inadequacy and defective erythropoiesis on the following health conditions and diseases.
Iron is critical for the development of the central nervous system, and iron deficiency is thought to be especially detrimental during the prenatal and early postnatal periods. Iron-dependent enzymes are required for nerve myelination, neurotransmitter synthesis, and normal neuronal energy metabolism (56). Most observational studies have found relationships between iron deficiency — with or without anemia — in children and poor cognitive development, poor school achievement, and abnormal behavior patterns (reviewed in 37). Whether psychomotor and mental deficits may be attributed to the lack of iron, only, or to a combination effect of iron deficiency and low hemoglobin concentrations — like in iron-deficiency anemia and anemia of inflammation — in early childhood remains unclear (14).
A recent systematic review of six small placebo-controlled trials (published between 1978 and 1989) in children with iron-deficiency anemia younger than 27 months found no convincing evidence that iron therapy (for less than 11 days) had any consistent effect on measures of psychomotor and mental development within 30 days of treatment initiation (57). Only one randomized, double-blind trial in anemic, iron-deficient infants examined the impact of iron therapy for four months and found a significant benefit on indices of cognitive development that needs to be further confirmed (58). A review of five randomized controlled trials in non-anemic, iron-deficient infants (0-9 months old) suggested an improvement in psychomotor (but not mental) development throughout the first 18 months of life (59). Iron supplementation in early infancy (4 to 6 months) also failed to demonstrate any long-term effect on cognitive performance and school performance at the age of 9 years compared to placebo (60). At present, evidence supporting any benefits of iron therapy on neurodevelopment outcomes in infants with iron deficiency, with or without anemia, remains limited.
Iron therapy might be more effective at improving cognitive outcomes in older children with anemia and/or iron deficiency. A systematic review of 17 randomized controlled trials found that iron supplementation had no effect on mental development of children under the age of 27 months but modestly improved scores of mental development in children over seven years of age (61). A more recent meta-analysis of randomized controlled trials in children older than six years, adolescents, and women with iron deficiency, anemia, or iron-deficiency anemia suggested that supplemental iron could improve attention and concentration irrespective of participants’ iron status (62). A potential improvement in IQ measures with iron therapy was also reported in anemic participants regardless of their iron status. No additional benefits were observed regarding measures of memory performance, psychomotor function, and school achievements.
Alterations in brain functions due to iron deficiency are likely to be resistant to iron therapy when they occur in early childhood. Long-term consequences of early life iron deficiency may include poor socioeconomic achievements and increased risk of certain psychopathologies, including anxiety, depression, and schizophrenia (56).
Epidemiological studies provide strong evidence of an association between severe anemia in pregnant women and adverse pregnancy outcomes, such as low birth weight, preterm birth, and neonatal and maternal mortality (63). Although iron deficiency can be a major contributing factor to severe anemia, evidence that iron-deficiency anemia causes poor pregnancy outcomes is still lacking. In addition, iron supplementation during pregnancy was shown to improve iron status and hematological parameters in women but failed to significantly reduce adverse pregnancy outcomes, including low birth weight and/or prematurity, neonatal death, and congenital anomalies (64). Moreover, routine supplementation during pregnancy had no effect on the length of gestation or newborn Apgar scores (40). Nevertheless, most experts consider the control of maternal anemia to be an important part of prenatal health care, and the IOM recommends screening for anemia in each trimester of pregnancy (65).
The requirement for iron is greatly increased in the second and third trimesters, and the RDA for pregnant women is 27 mg/day of iron (see The Recommended Dietary Allowance) (25). The American College of Obstetricians and Gynecologists recommend screening all pregnant women for anemia and advise iron supplementation when required (66). Nonetheless, the US Preventive Services Task Force (40) and the American Academy of Family Physicians (67) consider that evidence is lacking to evaluate the harms and benefits of screening for iron-deficiency anemia and supplementing with iron during pregnancy.
In malaria-endemic regions, however, iron supplementation may improve pregnancy outcomes when provided in conjunction with measures of prevention and management of malaria. Two recent randomized, placebo-controlled trials failed to find an increased risk of malaria infection in both iron-deficient and iron-replete pregnant women supplemented with iron, supporting the use of universal iron supplementation in malaria-endemic countries that adopt malaria intermittent preventive treatment (IPT) (68, 69).
Children who are chronically exposed to lead, even in small amounts, are more likely to develop learning disabilities, behavioral problems, and have low IQs. Deficits in growth and neurologic development may occur in the infants of women exposed to lead during pregnancy and lactation. In adults, lead toxicity may result in kidney damage and high blood pressure. Although the use of lead in paint products, gasoline, and food cans has been discontinued in the US, lead toxicity continues to be a significant health problem, especially in children living in inner cities (70). In 2012, the US Centers for Disease Control and Prevention set the reference value for blood lead concentration at 5 micrograms per deciliter (μg/dL) to identify children at risk. Yet, there is no known blood lead concentration below which children are 100% safe (71).
Iron deficiency and lead poisoning share a number of the same risk factors, including low socioeconomic status, ethnic minority groups, and residence in urban areas. Iron deficiency may increase the risk of lead poisoning in children, especially by increasing the intestinal absorption of lead via the DMT1 intestinal transporter (72). However, the use of iron supplementation in lead poisoning might be reserved for children who are truly iron deficient or for iron-replete children with chronic lead exposure (e.g., living in lead-exposed housing) (72).
Restless legs syndrome (RLS; also called Willis-Ekbom disease) is a neurologic movement disorder of unknown etiology. People with RLS experience unpleasant sensations resulting in an irresistible urge to move their legs and transient relief with movement. These sensations are more common at rest and often interfere with sleep (73). The prevalence of RLS is higher in women than in men and increases with age (74). This syndrome appears to be inherited in about 50% of patients but has also been related to chronic kidney failure (73). Iron deficiency may be involved in RLS development, possibly by affecting the activity of tyrosine hydroxylase, a rate limiting iron-dependent enzyme in the synthesis of the neurotransmitter, dopamine (74). The management of RLS includes iron therapy and the use of drugs like dopamine agonists (73). Current clinical evidence is insufficient to evaluate whether iron therapy may help relieve some RLS symptoms (74). Yet, the Medical Advisory Board of the Willis-Ekbom Disease Syndrome Foundation suggests that iron status should be assessed in all patients with RLS, and iron therapy be attempted on a case-by-case basis in those who might benefit from it (73).
The amount of iron in food or supplements that is absorbed and used by the body is influenced by the iron nutritional status of the individual and whether or not the iron is in the form of heme. Because it is absorbed by a different mechanism than nonheme iron, heme iron is more readily absorbed and its absorption is less affected by other dietary factors (2). In an attempt to improve body iron status, iron absorption is enhanced in individuals who are anemic or iron deficient compared to iron-replete individuals.
Heme iron comes mainly from hemoglobin and myoglobin in meat, poultry, and fish. Although heme iron accounts for only 10%-15% of the iron found in the diet, it may provide up to one-third of total absorbed dietary iron (54). The absorption of heme iron is less influenced by other dietary factors than that of nonheme iron (27).
Plants, dairy products, meat, and iron salts added to food and supplements are all sources of nonheme iron. The absorption of nonheme iron is strongly influenced by enhancers and inhibitors present in the same meal (27).
Enhancers of nonheme iron absorption
Inhibitors of nonheme iron absorption
National surveys in the US have indicated that the average dietary intake of iron is 16 to 18 mg/day in men, 12 mg/day in pre- and postmenopausal women, and about 15 mg/day in pregnant women (25). Thus, the majority of premenopausal and pregnant women in the US consume less than the RDA for iron, and many men consume more than the RDA (see The Recommended Dietary Allowance). In the US, most grain products are fortified with nonheme iron. The iron content of some relatively iron-rich foods is listed in milligrams (mg) in Table 2. For more information on the nutrient content of specific foods, search USDA's FoodData Central.
Iron supplements are indicated for the prevention and treatment of iron deficiency and iron deficiency anemia. Individuals who are not at risk of iron deficiency (e.g., adult men and postmenopausal women) should not take iron supplements without an appropriate medical evaluation. A number of iron supplements are available, and different forms provide different proportions of elemental iron. Ferrous sulfate heptahydrate is 20% elemental iron, ferrous sulfate monohydrate is 33% elemental iron, ferrous gluconate is 12% elemental iron, and ferrous fumarate is 33% elemental iron. If not stated otherwise, the iron discussed in this article is elemental iron.
Deregulation of intestinal iron absorption will result in iron overload because the body cannot excrete excess iron (2). However, iron overload due to prolonged iron supplementation is very rare in healthy individuals without a genetic predisposition. Several genetic disorders may lead to pathological accumulation of iron in the body despite normal iron intake. Supplementation of individuals who are not iron deficient should be avoided due to the frequency of undetected inherited diseases and recent concerns about the more subtle effects of chronic excess iron intake (see Diseases associated with iron overload).
Hereditary hemochromatosis (HH) refers to late-onset, autosomal recessive disorders of iron metabolism that result in iron accumulation in the liver, heart, and other tissues. Disorders may lead to cirrhosis of the liver, diabetes mellitus, cardiomyopathy (heart muscle damage), hypogonadism, arthropathy (joint problems), and increased skin pigmentation (reviewed in 78). There are four main types of HH, which are classified according to the specific gene that is mutated. The most common type of HH, called Type 1 or HFE-related HH, results from mutations in the HFE gene (79, 80). The majority of Type 1 HH cases are homozygous for mutation C282Y G>A (rs1800560) in the HFE gene. Another mutation found in 4% of patients with Type 1 HH is H63D C>G (rs1799945) in the HFE gene. The protein encoded by the HFE gene is thought to play a role in regulating intestinal absorption of dietary iron and with sensing the body’s iron stores (81). HFE gene mutations are associated with an increased cellular uptake of iron. With a typical disease onset before age 30, juvenile hemochromatosis (HH Type 2) is much rarer than Type 1 HH and results from genetic mutations affecting either hemojuvelin (Type 2A) or hepcidin (Type 2B) function (82). HH Type 3 results from mutations in the transferrin receptor 2 gene (TFR2), and HH Type 4 (also called Ferroportin disease) results from mutations in the gene encoding ferroportin-1 (SLC40A1), a protein important in the export of iron from cells (see Regulation). Type 4 HH is the second most common inherited iron overload disorder after Type 1 HH (78).
Iron overload in HH is treated by phlebotomy, the removal of 500 mL of blood at a time, at intervals determined by the severity of the iron overload. Chelation therapy is an alternate option to deplete iron in HH patients who cannot undergo phlebotomy treatment. Individuals with HH are advised to avoid supplemental iron, but generally not told to avoid iron-rich food. High-dose vitamin C regimens may worsen iron overload in patients with HH (75). Alcohol consumption is strongly discouraged due to the increased risk of cirrhosis of the liver (83). Genetic testing, which requires a blood sample, is available for those who may be at risk for HH, for example, individuals with a family history of hemochromatosis.
Other genetic disorders leading to iron overload include aceruloplasminemia, hypotransferrinemia, Friedreich’s ataxia, and porphyria cutanea tarda (2).
Iron overload may develop in individuals with severe hereditary anemias that are not caused by iron deficiency. Excessive dietary absorption of iron may occur in response to the body's continued efforts to form red blood cells. Beta-thalassemia is characterized by defective hemoglobin A synthesis due to mutations in the β-globin gene. Patients affected by thalassemia intermedia do not require blood transfusion as do those affected by the most severe form of the disease (called thalassemia major), yet they develop iron overload due to increased intestinal iron absorption (84). Other anemic patients at risk of iron overload include those with sideroblastic anemia, hemolytic anemia, pyruvate kinase deficiency, and thalassemia major, especially because they are treated with numerous transfusions. Patients with hereditary spherocytosis and thalassemia minor do not usually develop iron overload unless they are misdiagnosed as having iron deficiency and treated with large doses of iron over many years. Iron overload has also been associated with hemodialysis and chronic liver diseases (metabolic, viral, and alcoholic) (2).
Toxic iron deposition in vital organs in hereditary hemochromatosis (HH) has been associated with an increased incidence of liver cancer, type 2 diabetes mellitus, and neurodegenerative disease. Iron overload might also increase the risk of chronic disease in HH-free individuals. Nevertheless, whether iron tissue accumulation in those unaffected by genetic disorders is due to high dietary iron intakes is not yet fully understood (1).
Hereditary hemochromatosis that is characterized by abnormal hepatic iron accumulation is a risk factor for liver cancer (hepatocellular carcinoma; HCC). Iron accumulation is thought to function as a carcinogen by increasing oxidative stress that causes damage to lipids, proteins, and DNA. A meta-analysis of nine observational studies found an increased HCC risk with the C282Y mutation in the HFE gene of healthy participants and patients with chronic liver disease (see Iron Overload) (85). Other meta-analyses have reported associations between HFE gene mutations C282Y and H63D and increased risks of overall cancer (86, 87). However, studies reporting on HFE gene mutations and risk of cancer at extra-hepatic sites are rather scarce and/or inconsistent. Some, but not all, observational studies found significant associations between the C282Y mutation and risk of colorectal (88), breast (88, 89), and epithelial ovarian cancer (90). The presence of the H63D mutation in the HFE gene was linked to an increased risk of leukemia (91, 92) and gastric cancer (93).
Whether high dietary iron could increase the risk of cancer in individuals without hemochromatosis has also been investigated. The consumption of red or processed meat (but not white meat), rich in heme iron, has been linked to an increased risk of colorectal cancer (CRC) (94). Exposure to carcinogenic compounds (called heterocyclic amines) generated when meat is cooked at high temperatures and to carcinogenic N-nitroso compounds formed in the gastrointestinal tract following consumption of red and processed meat may explain such an association (95). Several meta-analyses of observational studies have also suggested a potential association of heme iron in red meat with CRC (96-98). This has been explained by an increased exposure of colonic cells to potentially damaging N-nitroso compounds and lipid peroxidation end-products derived from heme iron-catalyzed reactions (99). Further, recent results from the large European Prospective Investigation into Cancer and Nutrition (EPIC) study suggested a higher risk of esophageal adenocarcinoma with high intakes of red/processed meat and heme iron (100).
Experimental studies have suggested a role for iron-induced oxidative stress in vessel wall damage and the development of atherosclerosis, which underlies most forms of cardiovascular disease (101). However, epidemiological studies of iron nutritional status and cardiovascular disease in humans have yielded conflicting results. A recent systematic review and meta-analysis of 17 prospective cohort studies in 156,427 participants (9,236 cases of coronary heart disease [CHD] or myocardial infarction [MI]) did not find evidence to support the existence of strong associations between a number of different measures of iron status and CHD/MI (102). Only individuals in the highest versus lowest tertile of serum transferrin saturation exhibited an 18% lower incidence of CHD/MI (102). Another meta-analysis of 21 prospective studies found serum transferrin saturation and serum iron to be inversely associated with the risk of CHD. However, the authors noted that most studies failed to adjust for the confounding effects of inflammation (103). The review also reported an inverse association between CHD incidence and total dietary iron intake, but dietary heme iron was positively associated with CHD incidence (103). Although the relationship between iron stores and CHD/MI requires further clarification, it would be prudent for those who are not at risk of iron deficiency (e.g., adult men and postmenopausal women) to avoid excess iron intake (see the LPI Rx for Health).
Individuals with hereditary hemochromatosis (HH) are known to be at a heightened risk of developing type 2 diabetes mellitus (104). Increasing evidence also suggests a role for iron excess in the pathogenesis of type 2 diabetes independent of hemochromatosis. Cross-sectional, case-control, and prospective cohort studies have reported an increased risk of type 2 diabetes (105) and metabolic syndrome (106) with high versus low ferritin concentrations (reflecting iron body stores) after adjustment for inflammation. It is currently unclear how other indices of iron status relate to the risk of type 2 diabetes (107-110). Iron overload-induced oxidative stress in patients with HH is thought to damage pancreatic β-cells and impair insulin secretion. In subjects free of HH, iron excess might damage the liver, interfering with glucose metabolism and triggering insulin resistance, rather than impair β-cell function (111, 112). Iron removal by phlebotomy has been shown to improve metabolic parameters in subjects with type 2 diabetes (113) and metabolic syndrome (114). Additional randomized controlled trials are needed to determine whether lowering body stores of iron will aid in the prevention of type 2 diabetes and metabolic syndrome.
Iron is required for normal brain and nerve function through its involvement in cellular metabolism, as well as in the synthesis of neurotransmitters and myelin. Deregulation of iron homeostasis has been observed in a number of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease) (115-117). The abnormal accumulation of iron in the brain does not appear to be a result of increased dietary iron, but rather a disruption in the complex process of cellular iron regulation (117). Brain iron accumulation can result in increased oxidative stress, and the brain is particularly susceptible to oxidative damage. Mechanisms behind the disruption of iron homeostasis in the brain of patients affected by neurodegenerative disease are actively being investigated. For example, studies using genetically modified mouse models indicated that the suppression of amyloid precursor protein (APP) expression by upstream nitric oxide (NO) elevation (11) or loss of Tau protein (12) could impair neuronal export of iron and lead to iron accumulation in specific brain regions affected in Parkinson’s disease. A pilot, double-blind, placebo-controlled trial in patients with early-stage Parkinson’s disease demonstrated oral administration of the iron chelator, deferiprone, for 12 months reduced iron deposition in the part of the brain called substantia nigra and improved motor performance without compromising systemic iron homeostasis (118, 119).
Accidental overdose of iron-containing products is the single largest cause of poisoning fatalities in children under six years of age. Although the oral lethal dose of elemental iron is approximately 180 to 250 mg/kg of body weight, considerably less has been fatal. Symptoms of acute toxicity may occur with iron doses of 20 to 60 mg/kg of body weight. Iron overdose is an emergency situation because the severity of iron toxicity is related to the amount of elemental iron absorbed. Acute iron poisoning produces symptoms in four stages: (1) Within one to six hours of ingestion, symptoms may include nausea, vomiting, abdominal pain, tarry stools, lethargy, weak and rapid pulse, low blood pressure, fever, difficulty breathing, and coma; (2) If not immediately fatal, symptoms may subside for about 24 hours; (3) Symptoms may return 12 to 48 hours after iron ingestion and may include serious signs of failure in the following organ systems: cardiovascular, kidney, liver, hematologic (blood), and central nervous system; and (4) Long-term damage to the central nervous system, liver (cirrhosis), and stomach may develop two to six weeks after ingestion (25, 120).
At therapeutic levels used to treat iron deficiency, iron supplements may cause gastrointestinal irritation, nausea, vomiting, diarrhea, or constipation. Stools will often appear darker in color. Iron-containing liquids can temporarily stain teeth, but diluting the liquid helps to prevent this effect (120). Taking iron supplements with food instead of on an empty stomach may relieve gastrointestinal effects. The Food and Nutrition Board (FNB) of the Institute of Medicine based the tolerable upper intake level (UL) for iron on the prevention of gastrointestinal distress (Table 3). The UL for adolescents (14-18 years) and adults, including pregnant and breast-feeding women, is 45 mg/day. It should be noted that the UL is not meant to apply to individuals being treated with iron under close medical supervision. Individuals with hereditary hemochromatosis or other conditions of iron overload, as well as individuals with alcoholic cirrhosis and other liver diseases, may experience adverse effects at iron intake levels below the UL (25).
| Age Group | UL (mg/day) |
|---|---|
| Infants 0-12 months | 40 |
| Children 1-13 years | 40 |
| Adolescents 14-18 years | 45 |
| Adults 19 years and older | 45 |
Medications that decrease stomach acidity, such as antacids, histamine (H2) receptor antagonists (e.g., cimetidine, ranitidine), and proton-pump inhibitors (e.g., omeprazole, lansoprazole), may impair iron absorption. Taking iron supplements at the same time as the following medications may result in decreased absorption and efficacy of the medication: carbidopa and levodopa (Sinemet), levothyroxine (Synthroid, Levoxyl), methyldopa (Aldomet), penicillamine (Cuprimine, Depen), quinolones, tetracyclines, and bisphosphonates (120). Therefore, it is best to take these medications two hours apart from iron supplements. Cholestyramine (Questran) and colestipol (Colestid), used to lower blood cholesterol concentrations, should also be taken at least four hours apart from iron supplements because they may interfere with iron absorption (121).
Despite the critical functions of iron in the immune response, the nature of the relationship between iron status and susceptibility to infection, especially with respect to malaria, has been controversial. Because iron withholding is a recognized defense mechanism against pathogens (see Iron withholding defense during infection), concerns have been raised regarding the safety of iron supplementation, especially in iron-replete children living in malaria-endemic regions (122).
Iron supplementation of children residing in the tropics has been associated with increased risk of clinical malaria and other infections like pneumonia (123, 124). A randomized controlled trial in 24,076 children (ages, 1-35 months) living in a malaria-endemic region of eastern Africa (Tanzania) investigated the effects of supplemental iron and folic acid, with or without zinc, compared to the effects of zinc alone or a placebo, on all-cause mortality and hospital admissions (125). The administration of iron, folic acid, and/or zinc was found to increase the risk of serious adverse effects, hospital admission, and death, and was therefore prematurely halted. Further analyses of the trial revealed that iron-replete children were more likely than iron deficient-children (with or without anemia) to be at risk of adverse effects following iron supplementation (125). Such a potential risk of adverse effects with routine iron supplementation was not observed in preschool children in settings without malaria (southern Nepal) (126).
A recent review of 35 trials indicated that iron supplementation did not increase the risk of clinical malaria or other parasitic diseases, infections, and all-cause mortality in children living in malaria-endemic regions in which prevention and management of malaria are available (127). Moreover, a pooled analysis of three high-quality trials demonstrated that supplemental iron combined with anti-malarial treatment protected children against clinical malaria and improved hematological parameters (127). The World Health Organization (WHO) currently recommends the provision of iron supplementation in infants and children, together with measures of malaria prevention, diagnosis, and treatment in malaria-endemic areas (128).
Following the RDA for iron should provide sufficient iron to prevent deficiency without causing adverse effects in most individuals. Although sufficient iron can be obtained through a varied diet, a considerable number of people do not consume adequate iron to prevent deficiency. A multivitamin/mineral supplement containing 100% of the daily value (DV) for iron provides 18 mg of elemental iron. While this amount of iron may be beneficial for premenopausal women, it is well above the RDA for men and postmenopausal women.
Since hereditary hemochromatosis is not uncommon and the effects of long-term dietary iron excess on chronic disease risk are not yet clear, men and postmenopausal women who are not at risk of iron deficiency should take a multivitamin/mineral supplement without iron. A number of multivitamins formulated specifically for men or those over 50 years of age do not contain iron.
Moderately elevated iron stores might be much more common than iron deficiency in middle-age and older individuals (129). Thus, older adults should not generally take nutritional supplements containing iron unless they have been diagnosed with iron deficiency. Moreover, it is extremely important to determine the underlying cause of the iron deficiency, rather than simply treating it with iron supplements (see The Recommended Dietary Allowance).
Originally written in 2001 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in March 2003 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in January 2006 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in August 2009 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in April 2016 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in May 2016 by:
Marianne Wessling-Resnick, Ph.D.
Professor of Nutritional Biochemistry
Department of Genetics and Complex Diseases
Harvard T.H. Chan School of Public Health
The 2016 update of this article was underwritten, in part, by a grant from Bayer Consumer Care AG, Basel, Switzerland.
Copyright 2001-2024 Linus Pauling Institute
1. Aggett PJ. Iron. In: Erdman JWJ, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames: Wiley-Blackwell; 2012:506-520.
2. Wessling-Resnick M. Iron. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2014:176-188.
3. Winter WE, Bazydlo LA, Harris NS. The molecular biology of human iron metabolism. Lab Med. 2014;45(2):92-102. (PubMed)
4. Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212(Pt 10):1423-1428. (PubMed)
5. Salminen A, Kauppinen A, Kaarniranta K. 2-Oxoglutarate-dependent dioxygenases are sensors of energy metabolism, oxygen availability, and iron homeostasis: potential role in the regulation of aging process. Cell Mol Life Sci. 2015;72(20):3897-3914. (PubMed)
6. Zhang C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. 2014;5(10):750-760. (PubMed)
7. Anderson GJ, Darshan D, Wilkins SJ, Frazer DM. Regulation of systemic iron homeostasis: how the body responds to changes in iron demand. Biometals. 2007;20(3-4):665-674. (PubMed)
8. Fleming MD. The regulation of hepcidin and its effects on systemic and cellular iron metabolism. Hematology Am Soc Hematol Educ Program. 2008:151-158. (PubMed)
9. Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet. 2012;112(3):391-400. (PubMed)
10. Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. (PubMed)
11. Ayton S, Lei P, Hare DJ, et al. Parkinson's disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci. 2015;35(8):3591-3597. (PubMed)
12. Lei P, Ayton S, Finkelstein DI, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18(2):291-295. (PubMed)
13. Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2001;85 Suppl 2:S75-80. (PubMed)
14. Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126(5):1040-1050. (PubMed)
15. Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002;56(4):271-281. (PubMed)
16. Allen LH. Iron supplements: scientific issues concerning efficacy and implications for research and programs. J Nutr. 2002;132(4 Suppl):813S-819S. (PubMed)
17. Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. 1993;342(8883):1325-1328. (PubMed)
18. Jang JT, Green JB, Beard JL, Green MH. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J Nutr. 2000;130(5):1291-1296. (PubMed)
19. Rosales FJ, Jang JT, Pinero DJ, Erikson KM, Beard JL, Ross AC. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr. 1999;129(6):1223-1228. (PubMed)
20. Turnlund JR. Copper. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:286-299.
21. Thackeray EW, Sanderson SO, Fox JC, Kumar N. Hepatic iron overload or cirrhosis may occur in acquired copper deficiency and is likely mediated by hypoceruloplasminemia. J Clin Gastroenterol. 2011;45(2):153-158. (PubMed)
22. Videt-Gibou D, Belliard S, Bardou-Jacquet E, et al. Iron excess treatable by copper supplementation in acquired aceruloplasminemia: a new form of secondary human iron overload? Blood. 2009;114(11):2360-2361. (PubMed)
23. Food and Nutrition Board, Institute of Medicine. Copper. Dietary Reference Intakes for Vitamin A, Vitamin K, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C.: National Academy Press; 2001:224-257. (National Academy Press)
24. Kelkitli E, Ozturk N, Aslan NA, et al. Serum zinc levels in patients with iron deficiency anemia and its association with symptoms of iron deficiency anemia. Ann Hematol. 2016;95(5):751-756. (PubMed)
25. Food and Nutrition Board, Institute of Medicine. Iron. Dietary Reference Intakes for Vitamin A, Vitamin K, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C.: National Academy Press; 2001:290-393. (National Academy Press)
26. Lynch SR. Interaction of iron with other nutrients. Nutr Rev. 1997;55(4):102-110. (PubMed)
27. Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91(5):1461S-1467S. (PubMed)
28. Weaver CM. Calcium. In: Erdman JJ, Macdonald I, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed: John Wiley & Sons, Inc.; 2012:434-446.
29. Zimmermann MB. The influence of iron status on iodine utilization and thyroid function. Annu Rev Nutr. 2006;26:367-389. (PubMed)
30. Hess SY. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: the evidence from human studies. Best Pract Res Clin Endocrinol Metab. 2010;24(1):117-132. (PubMed)
31. Hess SY, Zimmermann MB, Adou P, Torresani T, Hurrell RF. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Cote d'Ivoire. Am J Clin Nutr. 2002;75(4):743-748. (PubMed)
32. World Health Organization, United Nations Children's Fund, United Nations University. Iron deficiency anaemia: assessment, prevention and control - A guide for programme managers 2001.
33. Global Burden of Disease Pediatrics C, Kyu HH, Pinho C, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;170(3):267-287. (PubMed)
34. Wang M. Iron deficiency and other types of anemia in infants and children. Am Fam Physician. 2016;93(4):270-278. (PubMed)
35. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2S-2):568S-579S; discussion 580S. (PubMed)
36. Changela K, Haeri NS, Krishnaiah M, Reddy M. Plummer-Vinson syndrome with proximal esophageal web. J Gastrointest Surg. 2015;20(5):1074-1075. (PubMed)
37. Jauregui-Lobera I. Iron deficiency and cognitive functions. Neuropsychiatr Dis Treat. 2014;10:2087-2095. (PubMed)
38. Lee GR. Disorders of iron metabolism and heme synthesis. In: Lee GR, Foerster J, Paraskevas F, Greer JP, Rogers GM, eds. Wintrobe's Clinical Hematology. Baltimore: Williams and Wilkins; 1999:979-1070.
39. McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Evid Based Child Health. 2014;9(2):303-397. (PubMed)
40. Siu AL, Force USPST. Screening for iron deficiency anemia in young children: USPSTF recommendation statement. Pediatrics. 2015;136(4):746-752. (PubMed)
41. Miller EM. Iron status and reproduction in US women: National Health and Nutrition Examination Survey, 1999-2006. PLoS One. 2014;9(11):e112216. (PubMed)
42. Brody T. Nutritional Biochemistry. 2nd ed. San Diego: Academic Press; 1999.
43. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011;93(6):1312-1320. (PubMed)
44. Khuroo MS, Khuroo MS, Khuroo NS. Trichuris dysentery syndrome: a common cause of chronic iron deficiency anemia in adults in an endemic area (with videos). Gastrointest Endosc. 2010;71(1):200-204. (PubMed)
45. Brittenham GM. Iron deficiency in whole blood donors. Transfusion. 2011;51(3):458-461. (PubMed)
46. Li H, Condon F, Kessler D, et al. Evidence of relative iron deficiency in platelet- and plasma-pheresis donors correlates with donation frequency. J Clin Apher. 2016; doi: 10.1002/jca.21448. [Epub ahead of print]. (PubMed)
47. Hershko C, Skikne B. Pathogenesis and management of iron deficiency anemia: emerging role of celiac disease, helicobacter pylori, and autoimmune gastritis. Semin Hematol. 2009;46(4):339-350. (PubMed)
48. Mahadov S, Green PH. Celiac disease: a challenge for all physicians. Gastroenterol Hepatol (N Y). 2011;7(8):554-556. (PubMed)
49. Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163(2):127-134. (PubMed)
50. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211-222. (PubMed)
51. Aron-Wisnewsky J, Verger EO, Bounaix C, et al. Nutritional and Protein Deficiencies in the Short Term following Both Gastric Bypass and Gastric Banding. PLoS One. 2016;11(2):e0149588. (PubMed)
52. Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J. Iron deficiency in obese postmenopausal women. Obesity (Silver Spring). 2006;14(10):1724-1730. (PubMed)
53. Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114(1):104-108. (PubMed)
54. Saunders AV, Craig WJ, Baines SK, Posen JS. Iron and vegetarian diets. Med J Aust. 2013;199(4 Suppl):S11-16. (PubMed)
55. Macdougall IC, Bircher AJ, Eckardt KU, et al. Iron management in chronic kidney disease: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2016;89(1):28-39. (PubMed)
56. Doom JR, Georgieff MK. Striking while the iron is hot: Understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep. 2014;2(4):291-298. (PubMed)
57. Wang B, Zhan S, Gong T, Lee L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev. 2013;6:CD001444. (PubMed)
58. Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341(8836):1-4. (PubMed)
59. Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. Am J Clin Nutr. 2010;91(6):1684-1690. (PubMed)
60. Pongcharoen T, DiGirolamo AM, Ramakrishnan U, Winichagoon P, Flores R, Martorell R. Long-term effects of iron and zinc supplementation during infancy on cognitive function at 9 y of age in northeast Thai children: a follow-up study. Am J Clin Nutr. 2011;93(3):636-643. (PubMed)
61. Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8(2):117-132. (PubMed)
62. Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J. 2010;9:4. (PubMed)
63. Burke RM, Leon JS, Suchdev PS. Identification, prevention and treatment of iron deficiency during the first 1000 days. Nutrients. 2014;6(10):4093-4114. (PubMed)
64. Pena-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;7:CD004736. (PubMed)
65. Institute of Medicine Committee on Preventive Services for Women; Board on Population Health and Public Health Practice. Clinical prevention services for women - closing the gaps: The National Academies Press; 2011. (The National Academies Press)
66. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201-207. (PubMed)
67. American Academy of Family Physicians. Clinical preventive service recommendation: iron deficiency anemia. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/iron-deficiency-anemia.html. Accessed 4/17/16.
68. Etheredge AJ, Premji Z, Gunaratna NS, et al. Iron supplementation in iron-replete and nonanemic pregnant women in Tanzania: a randomized clinical trial. JAMA Pediatr. 2015;169(10):947-955. (PubMed)
69. Mwangi MN, Roth JM, Smit MR, et al. Effect of daily antenatal iron supplementation on plasmodium infection in Kenyan women: a randomized clinical trial. JAMA. 2015;314(10):1009-1020. (PubMed)
70. Mielke HW, Gonzales C, Powell E, Mielke PW. Evolving from reactive to proactive medicine: community lead (Pb) and clinical disparities in pre- and post-Katrina New Orleans. Int J Environ Res Public Health. 2014;11(7):7482-7491. (PubMed)
71. Centers for Disease Control and Prevention. New blood lead level information. [Web page]. Available at: http://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm. Accessed 6/1/16.
72. Kwong WT, Friello P, Semba RD. Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ. 2004;330(1-3):21-37. (PubMed)
73. Silber MH, Becker PM, Earley C, Garcia-Borreguero D, Ondo WG, Medical Advisory Board of the Willis-Ekbom Disease F. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc. 2013;88(9):977-986. (PubMed)
74. Trotti LM, Bhadriraju S, Becker LA. Iron for restless legs syndrome. Cochrane Database Syst Rev. 2012;5:CD007834. (PubMed)
75. Johnston CS. Vitamin C. In: Erdman JWJ, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames: Wiley-Blackwell; 2012:248-260.
76. Morck TA, Lynch SR, Cook JD. Inhibition of food iron absorption by coffee. Am J Clin Nutr. 1983;37(3):416-420. (PubMed)
77. Natural Medicines. Iron: Interactions with Herbs & Supplements [professional monograph]; 2016. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 6/1/16.
78. Liu J, Pu C, Lang L, Qiao L, Abdullahi MA, Jiang C. Molecular pathogenesis of hereditary hemochromatosis. Histol Histopathol. 2016:11762. [Epub ahead of print]. (PubMed)
79. Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399-408. (PubMed)
80. Franchini M, Veneri D. Recent advances in hereditary hemochromatosis. Ann Hematol. 2005;84(6):347-352. (PubMed)
81. Ayonrinde OT, Milward EA, Chua AC, Trinder D, Olynyk JK. Clinical perspectives on hereditary hemochromatosis. Crit Rev Clin Lab Sci. 2008;45(5):451-484. (PubMed)
82. Wallace DF, Subramaniam VN. Non-HFE haemochromatosis. World J Gastroenterol. 2007;13(35):4690-4698. (PubMed)
83. Powell LW, Seckington RC, Deugnier Y. Haemochromatosis. Lancet. 2016; pii: S0140-6736(15)01315-X. doi: 10.1016/S0140-6736(15)01315-X. [Epub ahead of print]. (PubMed)
84. Oikonomidou PR, Casu C, Rivella S. New strategies to target iron metabolism for the treatment of beta thalassemia. Ann N Y Acad Sci. 2016; 1368(1):162-168. (PubMed)
85. Jin F, Qu LS, Shen XZ. Association between C282Y and H63D mutations of the HFE gene with hepatocellular carcinoma in European populations: a meta-analysis. J Exp Clin Cancer Res. 2010;29:18. (PubMed)
86. Shen LL, Gu DY, Zhao TT, Tang CJ, Xu Y, Chen JF. Implicating the H63D polymorphism in the HFE gene in increased incidence of solid cancers: a meta-analysis. Genet Mol Res. 2015;14(4):13735-13745. (PubMed)
87. Zhang M, Xiong H, Fang L, et al. Meta-Analysis of the Association between H63D and C282Y Polymorphisms in HFE and Cancer Risk. Asian Pac J Cancer Prev. 2015;16(11):4633-4639. (PubMed)
88. Osborne NJ, Gurrin LC, Allen KJ, et al. HFE C282Y homozygotes are at increased risk of breast and colorectal cancer. Hepatology. 2010;51(4):1311-1318. (PubMed)
89. Liu X, Lv C, Luan X, Lv M. C282Y polymorphism in the HFE gene is associated with risk of breast cancer. Tumour Biol. 2013;34(5):2759-2764. (PubMed)
90. Gannon PO, Medelci S, Le Page C, et al. Impact of hemochromatosis gene (HFE) mutations on epithelial ovarian cancer risk and prognosis. Int J Cancer. 2011;128(10):2326-2334. (PubMed)
91. Kennedy AE, Kamdar KY, Lupo PJ, et al. Examination of HFE associations with childhood leukemia risk and extension to other iron regulatory genes. Leuk Res. 2014;38(9):1055-1060. (PubMed)
92. Viola A, Pagano L, Laudati D, et al. HFE gene mutations in patients with acute leukemia. Leuk Lymphoma. 2006;47(11):2331-2334. (PubMed)
93. Agudo A, Bonet C, Sala N, et al. Hemochromatosis (HFE) gene mutations and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Carcinogenesis. 2013;34(6):1244-1250. (PubMed)
94. Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657-2664. (PubMed)
95. Cross AJ, Ferrucci LM, Risch A, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70(6):2406-2414. (PubMed)
96. Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila). 2011;4(2):177-184. (PubMed)
97. Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk--a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev. 2014;23(1):12-31. (PubMed)
98. Qiao L, Feng Y. Intakes of heme iron and zinc and colorectal cancer incidence: a meta-analysis of prospective studies. Cancer Causes Control. 2013;24(6):1175-1183. (PubMed)
99. Bastide NM, Chenni F, Audebert M, et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75(5):870-879. (PubMed)
100. Jakszyn P, Lujan-Barroso L, Agudo A, et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2013;133(11):2744-2750. (PubMed)
101. de Valk B, Marx JJ. Iron, atherosclerosis, and ischemic heart disease. Arch Intern Med. 1999;159(14):1542-1548. (PubMed)
102. Das De S, Krishna S, Jethwa A. Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis. 2015;238(2):296-303. (PubMed)
103. Hunnicutt J, He K, Xun P. Dietary iron intake and body iron stores are associated with risk of coronary heart disease in a meta-analysis of prospective cohort studies. J Nutr. 2014;144(3):359-366. (PubMed)
104. Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30(7):1926-1933. (PubMed)
105. Orban E, Schwab S, Thorand B, Huth C. Association of iron indices and type 2 diabetes: a meta-analysis of observational studies. Diabetes Metab Res Rev. 2014;30(5):372-394. (PubMed)
106. Abril-Ulloa V, Flores-Mateo G, Sola-Alberich R, Manuel-y-Keenoy B, Arija V. Ferritin levels and risk of metabolic syndrome: meta-analysis of observational studies. BMC Public Health. 2014;14:483. (PubMed)
107. Huth C, Beuerle S, Zierer A, et al. Biomarkers of iron metabolism are independently associated with impaired glucose metabolism and type 2 diabetes: the KORA F4 study. Eur J Endocrinol. 2015;173(5):643-653. (PubMed)
108. Montonen J, Boeing H, Steffen A, et al. Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia. 2012;55(10):2613-2621. (PubMed)
109. Podmore C, Meidtner K, Schulze MB, et al. The association of multiple biomarkers of iron metabolism and type 2 diabetes: the EPIC-InterAct study. Diabetes Care. 2016; 39(4):572-581. (PubMed)
110. Yeap BB, Divitini ML, Gunton JE, et al. Higher ferritin levels, but not serum iron or transferrin saturation, are associated with Type 2 diabetes mellitus in adult men and women free of genetic haemochromatosis. Clin Endocrinol (Oxf). 2015;82(4):525-532. (PubMed)
111. Fernandez-Real JM, McClain D, Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care. 2015;38(11):2169-2176. (PubMed)
112. Huang J, Karnchanasorn R, Ou HY, et al. Association of insulin resistance with serum ferritin and aminotransferases-iron hypothesis. World J Exp Med. 2015;5(4):232-243. (PubMed)
113. Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and β-cell function. Diabetes. 2002;51(4):1000-1004. (PubMed)
114. Houschyar KS, Ludtke R, Dobos GJ, et al. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Med. 2012;10:54. (PubMed)
115. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem. 2015; doi: 10.1111/jnc.13425. [Epub ahead of print]. (PubMed)
116. Kwan JY, Jeong SY, Van Gelderen P, et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One. 2012;7(4):e35241. (PubMed)
117. Wong BX, Duce JA. The iron regulatory capability of the major protein participants in prevalent neurodegenerative disorders. Front Pharmacol. 2014;5:81. (PubMed)
118. Devos D, Moreau C, Devedjian JC, et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal. 2014;21(2):195-210. (PubMed)
119. Grolez G, Moreau C, Sablonniere B, et al. Ceruloplasmin activity and iron chelation treatment of patients with Parkinson's disease. BMC Neurol. 2015;15:74. (PubMed)
120. Hendler SS, Rorvik DM. PDR for Nutritional Supplements. 2nd ed. Montvale: Thomson Reuters; 2008.
121. Natural Medicines. Iron: Interactions with Drugs [professional monograph]; 2016. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 6/1/16.
122. Wander K, Shell-Duncan B, McDade TW. Evaluation of iron deficiency as a nutritional adaptation to infectious disease: an evolutionary medicine perspective. Am J Hum Biol. 2009;21(2):172-179. (PubMed)
123. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131(2S-2):616S-633S; discussion 633S-635S. (PubMed)
124. van den Hombergh J, Dalderop E, Smit Y. Does iron therapy benefit children with severe malaria-associated anaemia? A clinical trial with 12 weeks supplementation of oral iron in young children from the Turiani Division, Tanzania. J Trop Pediatr. 1996;42(4):220-227. (PubMed)
125. Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133-143. (PubMed)
126. Tielsch JM, Khatry SK, Stoltzfus RJ, et al. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367(9505):144-152. (PubMed)
127. Neuberger A, Okebe J, Yahav D, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2016;2:CD006589. (PubMed)
128. World Health Organization. Guideline: daily iron supplementation in infants and children. Geneva: World Health Organization; 2016.
129. Fairweather-Tait SJ, Wawer AA, Gillings R, Jennings A, Myint PK. Iron status in the elderly. Mech Ageing Dev. 2014;136-137:22-28. (PubMed)