Vitamin E
Contents
Read the Nutrition Information Brief on vitamin E by Maret G. Traber, Ph.D.
Summary
- Naturally occurring vitamin E includes eight fat-soluble isoforms: α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol. Yet, the body preferentially uses α-tocopherol, and only α-tocopherol supplementation can reverse vitamin E deficiency symptoms. (More information)
- α-Tocopherol functions as a chain-breaking antioxidant, preventing the propagation of free radicals in membranes and plasma lipoproteins. α-Tocopherol is also likely involved in strengthening certain aspects of cell-mediated immunity. (More information)
- Vitamin E deficiency can be caused by fat malabsorption disorders or by genetic abnormalities that affect vitamin E transport. Severe deficiency symptoms include vitamin E deficiency-induced ataxia, peripheral neuropathy, muscle weakness, and damage to the retina of the eye. (More information)
- The current recommended dietary allowance (RDA) is 15 mg/day of α-tocopherol. It is estimated that more than 90% of Americans adults do not meet the estimated average requirement (EAR) of 12 mg/day of α-tocopherol. (More information)
- Randomized controlled trials investigating primary and/or secondary prevention of chronic diseases, such as cardiovascular disease, cancer, and cataracts, do not currently support a preventative effect of supplemental α-tocopherol. (More information)
- Limited clinical evidence suggests that vitamin E supplementation may be beneficial for managing age-related macular degeneration and fatty liver diseases secondary to type 2 diabetes mellitus. (More information)
- Supplementation with α-tocopherol was found to slow cognitive decline or loss of functional abilities in cognitively impaired subjects in some, but not all, clinical studies. (More information)
- Plant seeds, especially sunflower seeds, almonds, and hazelnuts, are rich sources of α-tocopherol such that many vegetable oils (e.g., olive oil and canola oil) also contain α-tocopherol. Other sources include tomato, avocado, spinach, asparagus, Swiss chard, and broccoli. (More information)
- High doses of supplemental α-tocopherol may interfere with the vitamin K-dependent blood clotting cascade and increase the risk of bleeding in individuals taking anticoagulant drugs. A tolerable upper intake level (UL) for α-tocopherol in adults is set at 1,000 mg/day and applies to all possible stereoisomers of α-tocopherol. (More information)
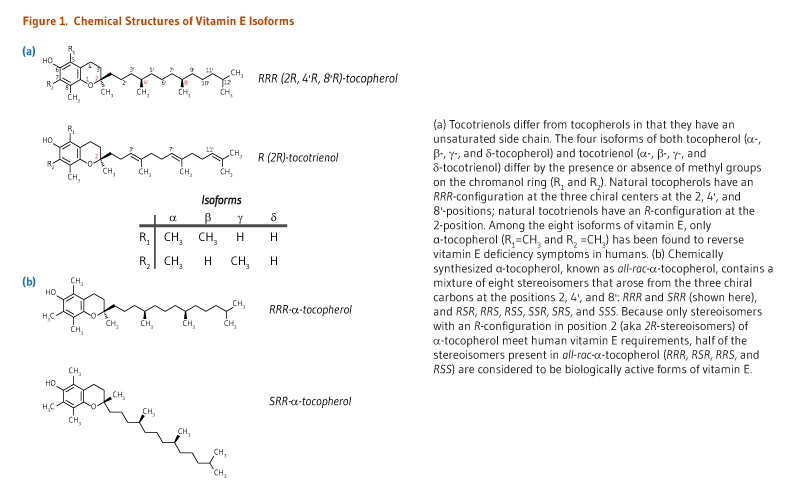
The term vitamin E describes a family of eight fat-soluble molecules with antioxidant activities: four tocopherol isoforms (α-, β-, γ-, and δ-tocopherol) and four tocotrienol isoforms (α-, β-, γ-, and δ-tocotrienol) (Figure 1). Only one form, α-tocopherol, meets human vitamin E requirements (see The RDA). In the human liver, α-tocopherol is the form of vitamin E that is preferentially bound to α-tocopherol transfer protein (α-TTP) and incorporated into lipoproteins that transport α-tocopherol in the blood for delivery to extrahepatic tissues. Therefore, it is the predominant form of vitamin E found in the blood and tissues (1). In addition, α-tocopherol appears to be the form of vitamin E with the greatest nutritional significance, such that it will be the primary topic of the following discussion.
Function
α-Tocopherol
Natural versus synthetic α-tocopherol
Natural α-tocopherol made by plants found in food has an RRR-configuration at the 2, 4’, and 8’-position of the α-tocopherol molecule (wrongly referred to as d-α-tocopherol) (see Figure 1). Chemically synthesized all-rac-α-tocopherol (all-racemic-α-tocopherol; incorrectly labeled dl-α-tocopherol) is a mixture of eight stereoisomers of α-tocopherol, which arose from the three chiral carbons at the 2, 4’, and 8’-positions: RRR-, RSR-, RRS-, RSS-, SRR-, SSR-, SRS-, and SSS-α-tocopherol (see Figure 1). While all stereoisomers have equal in vitro antioxidant activity, only the forms in the R-conformation at position 2 (noted 2R) meet the vitamin E requirements in humans (2).
Antioxidant activity
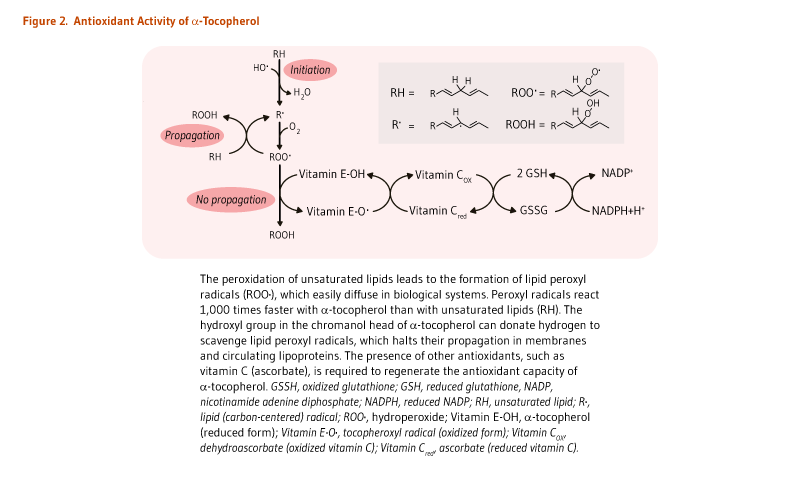
The main function of α-tocopherol in humans is that of a fat-soluble antioxidant. Fats, which are an integral part of all cell membranes, are vulnerable to damage through lipid peroxidation by free radicals. α-Tocopherol is uniquely suited to intercept peroxyl radicals and thus prevent a chain reaction of lipid oxidation (Figure 2). When a molecule of α-tocopherol neutralizes a free radical, it is oxidized and its antioxidant capacity is lost. Other antioxidants, such as vitamin C, are capable of regenerating the antioxidant capacity of α-tocopherol (Figure 2) (reviewed in 1).
Aside from maintaining the integrity of cell membranes throughout the body, α-tocopherol protects the fats in low-density lipoproteins (LDLs) from oxidation. Lipoproteins are particles composed of lipids and proteins that transport fats through the bloodstream. LDLs specifically transport cholesterol from the liver to the tissues of the body. Oxidized LDLs have been implicated in the development of cardiovascular disease (3).
Effects on cell-mediated immunity
Other functions of α-tocopherol are likely to be related to its antioxidant capacity (1). For instance, α-tocopherol can protect the physiological properties of lipid bilayer membranes and may influence the activity of membrane proteins and enzymes (4). In cell culture studies, α-tocopherol was found to improve the formation of an adhesive junction (known as immune synapse) between naïve T lymphocytes and antigen-presenting cells (APC), which eventually prompted T cell activation and proliferation (see Disease Prevention) (5, 6).
γ-Tocopherol and tocotrienols
Vitamin E forms other than α-tocopherol are also known to be potent antioxidants. Tocotrienols and γ-tocopherol are thought to be better scavengers of peroxyl radicals and reactive nitrogen species, respectively, than α-tocopherol (7). Yet, in the body, (1) α-tocopherol is preferentially retained in the liver by the binding to α-tocopherol transfer protein (α-TTP), which incorporates α-tocopherol into lipoproteins for delivery to extrahepatic tissues; and (2) forms of vitamin E other than α-tocopherol are actively metabolized and excreted. Hence, while γ-tocopherol is the most common form of vitamin E in the American diet (8), its plasma and tissue concentrations are generally significantly lower than those of α-tocopherol, and more γ-tocopherol is excreted in urine than α-tocopherol, suggesting less γ-tocopherol is needed for use by the body (1).
Studies conducted in vitro and in animals have indicated that γ-tocopherol and its major metabolite, γ-carboxyethyl hydroxychroman (γ-CEHC), may play a role in protecting the body from free radical-induced damage in various conditions of oxidative stress and inflammation (reviewed in 7). Limited intervention studies (highlighted in 7) have not convincingly demonstrated a potential anti-inflammatory effect of γ-tocopherol in humans. Yet, in two recent randomized, placebo-controlled studies, the supplementation of smokers with γ-tocopherol potentiated short-term benefits of smoking cessation (with or without nicotine replacement therapy) on vascular endothelial function (9, 10).
Numerous preclinical studies have also suggested that tocotrienols might be beneficial in the prevention of chronic diseases (11). For instance, tocotrienols (especially δ-tocotrienol) have shown greater anti-proliferative and pro-apoptotic effects than tocopherols in malignant cell lines (12). However, a number of factors, including dose, formulation, and type of study population, affect the bioavailability of tocotrienols and may undermine their putative efficacy in humans (13). There are currently no data available on the effectiveness of supplemental tocotrienols in humans (11).
Nutrient interactions
Dietary and circulating fatty acids
The mechanism of vitamin E digestion and uptake into intestinal cells (enterocytes) is unclear but requires bile acids and pancreatic enzymes, and the packaging along with dietary fat into chylomicrons. The efficiency of vitamin E absorption increases with the amount of fat in ingested food, such that vitamin E absorption from supplements is likely to be minimal with low-fat meals (14, 15).
In the circulation, all lipoproteins (i.e., VLDLs, LDLs, and HDLs) are involved in the transport and tissue distribution of α-tocopherol (1). Increased concentrations of lipids (cholesterol and triglycerides) in the blood have been correlated to higher serum α-tocopherol concentrations. However, if a high blood concentration of lipids is associated with a slower turnover of lipoproteins, then the distribution of α-tocopherol to tissues may be substantially altered (16).
Vitamin C
A few human studies using conditions of oxidative stress have demonstrated the importance of vitamin C (ascorbic acid) in the recycling of oxidized α-tocopherol back to its reduced state (see Figure 2). Oxidative stress caused by cigarette smoking accelerates the depletion of plasma α-tocopherol in smokers compared to nonsmokers (17). In a double-blind, placebo-controlled trial in 11 smokers and 13 nonsmokers given α-tocopherol and γ-tocopherol that was labeled with deuterium (hence traceable), supplementation with vitamin C reduced the rate of vitamin E loss in plasma, most probably by regenerating tocopheryl radicals back to nonoxidized forms (18).
Vitamin K
One study in adults with normal coagulation (clotting) status found that daily supplementation with 1,000 IU (670 mg) of RRR-α-tocopherol for 12 weeks decreased γ-carboxylation of prothrombin, a vitamin K-dependent factor in the coagulation cascade (19). Individuals taking anticoagulant drugs like warfarin and those who are vitamin K deficient should not take vitamin E supplements without medical supervision because of the increased risk of bleeding (see Safety) (20).
Deficiency
Causes
Severe vitamin E deficiency rarely occurs in humans but has been observed as a result of malnutrition (21). Severe vitamin E deficiency has been associated with specific genetic defects affecting the transport of α-tocopherol by α-tocopherol transfer protein (α-TTP) and lipoproteins. Vitamin E deficiency has also been observed in individuals with fat malabsorption syndromes, which impair the absorption of dietary fats and therefore fat-soluble vitamins like vitamin E (see Nutrient interactions) (21).
Symptoms
Severe vitamin E deficiency results mainly in neurologic symptoms, including impaired balance and coordination (spinocerebellar ataxia), injury to the sensory nerves (peripheral neuropathy), muscle weakness (myopathy), and damage to the retina of the eye (retinopathy). For this reason, people who develop peripheral neuropathy, ataxia, or retinitis pigmentosa (RP) of unknown causes should be screened for vitamin E deficiency (21). The results of one randomized controlled trial in 601 patients with common forms of RP indicated that daily supplementation with 400 IU of all-rac-α-tocopherol (180 mg of RRR-α-tocopherol) modestly but significantly increased the loss of retinal function (22). In contrast, daily supplementation with 15,000 IU of vitamin A (4,500 μg RAE) significantly slowed the loss of retinal function over a period of four to six years, suggesting that patients with common forms of RP may benefit from long-term vitamin A supplementation but should avoid high-dose supplemental vitamin E.
Inherited defects in α-TTP are associated with a characteristic syndrome called AVED (Ataxia with Vitamin E Deficiency). A recent case study reported that visual impairment in a middle-age patient with AVED was caused by both RP and early-onset macular degeneration (23). Supplementation with high-dose vitamin E (800-1,200 mg/day) is used to prevent neurologic deterioration in AVED subjects (21).
Moreover, the developing nervous system appears to be especially vulnerable to vitamin E deficiency. For instance, children with severe vitamin E deficiency at birth rapidly experience irreversible neurologic symptoms if not treated with vitamin E. In contrast, individuals who develop gastrointestinal disorders affecting vitamin E absorption in adulthood may not develop neurologic symptoms for 10-20 years (21). It should also be noted that neurologic symptoms caused by vitamin E deficiency have not been reported in healthy individuals who consume diets low in vitamin E.
Marginal deficiency
Although frank vitamin E deficiency is rare, marginal intake of vitamin E is relatively common. Between 1988 and 1994, the US National Health and Nutrition Examination Survey III (NHANES III) examined the dietary intake and blood concentrations of α-tocopherol in 16,295 adults. The study reported that about one-third of all participants had blood concentrations of α-tocopherol below 20 micromoles/liter (μmol/L) — a cutoff value chosen because of its initial association with an increased risk for cardiovascular disease (24). More recent data from 18,063 participants in NHANES 2003-2006 indicated an average dietary intake of α-tocopherol from food (including enriched and fortified sources) among Americans adults of 7.2 mg/day (25). This intake is well below the current recommended dietary allowance of 15 mg/day (see the RDA). At this level of dietary intake, more than 93% of American adults do not meet the estimated average requirement (EAR) of 12 mg/day for vitamin E (25). In addition, a recent nested case-control study in Bangladeshi women suggested that inadequate vitamin E status during early pregnancy may be associated with an increased risk of miscarriage (26).
Cigarette smoking is thought to increase the utilization of α-tocopherol such that smokers might be at increased risk of deficiency compared with nonsmokers (17). Also, the 19-year follow-up analysis of the Alpha-Tocopherol, Beta-Carotene cancer (ATBC) trial in older, male smokers indicated that participants in the highest versus lowest quintile of serum α-tocopherol concentrations (>31 μmol/L vs. <23 μmol/L) at baseline had reduced risks of total and cause-specific mortality (27).
It is not known whether marginal vitamin E deficiency increases the risk of chronic disease (1).
The Recommended Dietary Allowance (RDA)
The RDA for vitamin E was last revised by the Food and Nutrition Board of the US Institute of Medicine in 2000 (Table 1) (2). The RDA is based largely on the results of studies done in the 1950s in men fed vitamin E-deficient diets. In a test-tube analysis, vitamin E suppresses the breakdown of red blood cells (known as hemolysis) induced by hydrogen peroxide. Because hemolysis has also been reported in children with severe vitamin E deficiency, the preventive effect of vitamin E against oxidative damage-induced hemolysis was considered to be a clinically relevant in vitro analysis to assess vitamin E status. Importantly, this means that the latest RDA for vitamin E continues to be based on the prevention of deficiency symptoms rather than on health promotion and prevention of chronic disease.
The forms of α-tocopherol that meet the recommended intakes are RRR-α-tocopherol — the only naturally occurring form of vitamin E — and the three synthetic isomers, RRS-, RSR-, and RSS-α-tocopherol, which are found in nutritional supplements and fortified food.
Table 1 lists the RDA for α-tocopherol expressed in both milligrams (mg) and international units (IU).
| Life Stage | Age | Males | Females | ||
|---|---|---|---|---|---|
| mg/day | IU/day | mg/day | IU/day | ||
| Infants (AI) | 0-6 months |
4
|
6
|
4
|
6
|
| Infants (AI) | 7-12 months |
5
|
7.5
|
5
|
7.5
|
| Children | 1-3 years |
6
|
9
|
6
|
9
|
| Children | 4-8 years |
7
|
10.5
|
7.5
|
10.5
|
| Children | 9-13 years |
11
|
16.5
|
11
|
16.5
|
| Adolescents | 14-18 years |
15
|
22.5
|
15
|
22.5
|
| Adults | 19 years and older |
15
|
22.5
|
15
|
22.5
|
| Pregnancy | all ages |
-
|
-
|
15
|
22.5
|
| Breast-feeding | all ages |
-
|
-
|
19
|
28.5
|
|
*These recommended intakes are limited to 2R-stereoisomeric forms of α-tocopherol. |
|||||
Disease Prevention
Age-related deterioration of immune function
The natural age-related decline of the immune function is accompanied by an increased susceptibility to infections, a poorer response to immunization, and higher risks of developing cancers and autoimmune diseases. α-Tocopherol has been shown to enhance specifically the T cell-mediated immune response that declines with advancing age (reviewed in 28). T cell impaired response has been partly associated with a reduced capacity of naïve T cells to be activated during antigen presentation, and to produce interleukin-2 (IL-2) and proliferate as a result (6). However, very few studies have addressed the potential association between α-tocopherol and immune function in humans (28). In a small intervention study in older adults (mean age, 70 years), supplementation with 200 mg/day of all-rac-α-tocopherol (equivalent to 100 mg of RRR-α-tocopherol) for three months significantly improved natural killer (NK) cytotoxic activity, neutrophil chemotaxis, phagocytic response, and enhanced mitogen-induced lymphocyte proliferation and interleukin-2 (IL-2) production compared to baseline (29). In an earlier trial, daily supplementation of healthy older adults (≥65 years of age) with 200 mg of all-rac-α-tocopherol for 235 days also improved T lymphocyte-mediated immunity — as measured with the delayed-type hypersensitivity (DTH) skin test — and increased the production of antibodies in response to hepatitis B and tetanus vaccines (30).
Lower α-tocopherol doses failed to improve the DTH response compared to a placebo in another study in healthy participants (ages, 65-80 years) (31). A randomized, placebo-controlled trial in 617 nursing home residents (≥65 years of age) reported that daily supplementation with 200 IU of synthetic α-tocopherol (90 mg of RRR-α-tocopherol) for one year significantly lowered the risk of contracting upper respiratory tract infections, especially the common cold, but had no effect on lower respiratory tract (lung) infections (32). More research is needed to examine whether supplemental vitamin E might enhance immune function and reduce risk of infection in older adults.
Cardiovascular disease
Primary prevention: in healthy adults
Observational studies: Results of several large observational studies in both men and women have suggested an inverse relationship between vitamin E consumption and risk of myocardial infarction or death from heart disease. Each study had a prospective design that measured vitamin E intake in generally healthy people who were followed over a period of time to determine the onset of cardiovascular events and analyze the association between the exposure and the outcome(s). In two of the studies, individuals who consumed more than 7 mg/day of dietary α-tocopherol were 35% less likely to die from heart disease than those who consumed less than 3-5 mg/day of α-tocopherol (33, 34). Two other large studies observed a significantly reduced risk of heart disease only in women and men who consumed at least 100 IU (67 mg)/day of supplemental RRR-α-tocopherol (35, 36).
Intervention studies: A randomized, placebo-controlled, intervention trial in 39,876 women (aged ≥45 years) participating in the Women's Health Study (WHS) found that supplementation with 600 IU (400 mg) of RRR-α-tocopherol every other day for 10 years resulted in a 34% reduction in nonfatal myocardial infarction and a 49% reduction in cardiovascular-related deaths but only in women aged at least 65 years at baseline (representing 10% of study participants) (37). Further analyses of WHS data showed that women in the vitamin E arm of the study experienced a 21% reduction in risk of venous thromboembolism (VTE) compared to placebo: the reduction was of 12% in women younger than 55 years old, 26% in women aged 65 years and older, and 44% in women with a history of VTE (38, 39). Another large randomized controlled trial — the Physicians’ Health Study II (PHSII) — conducted in healthy middle-aged men found no significant effect of 400 IU of synthetic α-tocopherol (180 mg of RRR-α-tocopherol), given every other day for eight years, on the risk of major cardiovascular events in the entire cohort and in subgroup analyses (40). Besides, concerns were raised regarding a possible harmful effect of high-dose vitamin E supplementation on the risk of hemorrhagic stroke in this cohort (40).
Secondary prevention: in individuals with or at risk of cardiovascular disease
Conventional risk factors for cardiovascular disease (CVD) include cigarette smoking, physical inactivity, hypertension, dyslipidemia, and being overweight or obese. Other factors such as oxidative stress and inflammation are also thought to contribute to increasing CVD risk, especially in patients with chronic conditions like type 2 diabetes mellitus and chronic kidney disease. Although trials do not appear to support any cardiovascular benefit in healthy middle-aged and older subjects, vitamin E supplementation might help improve cardiovascular health and/or lower the risk of CVD in specific, higher risk subjects.
Observational studies: The presence of atherosclerotic plaques in arterial walls is one of the hallmarks of cardiovascular disease. Plaque rupture that causes blood clot formation is the usual cause of myocardial and cerebrovascular infarctions. The cross-sectional Asymptomatic Carotid Atherosclerosis Disease In Manfredonia (ACADIM) study in 640 at-risk individuals reported an inverse association between carotid intima-media thickness (CIMT) — a marker of atherosclerosis — and circulating concentrations of antioxidants, including vitamin E (41). However, other observational studies found no association between plasma vitamin E concentrations and CIMT (reviewed in 42).
Intervention studies: A small randomized controlled study assessing the effect of lipid-lowering drugs in men who had previously undergone a coronary artery bypass surgery found that the use of at least 100 IU/day (compared to less than 100 IU/day) of supplemental α-tocopherol (45 mg of RRR-α-tocopherol) was associated with reduced CIMT progression over a two-year period but only among participants in the placebo arm of the study (i.e., those who did not receive lipid-lowering drugs) (43). However, a recent meta-analysis of seven small placebo-controlled trials found little evidence that vitamin E supplementation may improve flow-mediated vascular dilation (FMD) of the brachial artery, a marker of vascular endothelial health that is adversely affected by CVD risk factors (44). In the Cambridge Heart AntiOxidant Study (CHAOS), a randomized, placebo-controlled intervention trial in 2,002 patients with coronary heart disease, daily supplementation with either 400 IU or 800 IU of synthetic α-tocopherol (180 mg or 360 mg of RRR-α-tocopherol) for a median 18 months dramatically reduced the occurrence of nonfatal myocardial infarctions by 77%. However, vitamin E supplementation did not significantly reduce total or cardiovascular-related deaths (45).
Another small trial in patients with renal failure — the Secondary Prevention with Antioxidants of cardiovascular disease in End-stage renal disease (SPACE) — found that supplementation with 800 IU (536 mg)/day of RRR-α-tocopherol for an average of 1.4 years significantly reduced the risk of myocardial infarction compared to placebo (46). A more recent randomized controlled study suggested that vitamin E supplementation may benefit a subgroup of patients with type 2 diabetes mellitus. The multicenter study by Milman et al. (47) was conducted in 1,434 type 2 diabetics (≥55 years old) carrying a specific variant of the haptoglobin protein (Hp), Hp2-2, which has a lower efficacy to bind and remove pro-oxidant, free hemoglobin from plasma, compared to Hp1-1 and Hp1-2 variants. The daily supplementation with 400 IU (268 mg) of RRR-α-tocopherol for 18 months resulted in a lower risk of myocardial infarction compared to placebo (47).
Other larger intervention trials conducted in cigarette smokers (the Alpha-Tocopherol, Beta-Carotene cancer prevention [ATBC] study (48)), in individuals at-risk of CVD (the Heart Outcomes Prevention Evaluation [HOPE]-The Ongoing Outcomes [HOPE-TOO study] (49)), or in patients who have suffered a myocardial infarction (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico-GISSI-prevenzione trial (50)) failed to find significant CVD risk reductions with α-tocopherol supplementation. Besides, potentially harmful effects of supplemental vitamin E were reported on the risk of hemorrhagic stroke in the ATBC trial and on the risk of heart failure in the HOPE and GISSI trials (see Safety) (48-50).
Cancer
Oxidative damage to DNA by free radicals can lead to mutations that may contribute to causing cancer (51). Because of its ability to neutralize free radicals, vitamin E has been suggested to possess anticancer activity by protecting cells against oxidative damage. Yet, several large prospective cohort studies have failed to find significant associations between vitamin E intake and the incidence of lung or breast cancer (2). More recently, the VITamins And Lifestyle (VITAL) study prospectively assessed the association between long-term use of supplemental vitamins (10-year intake) and the risk of lung cancer in a cohort of 77,126 men and women (52). No relationships were reported between intake of multivitamins, vitamin C, vitamin E, or folate and the risk of lung cancer. However, the use of supplemental vitamin E in current but not in former smokers was associated with an 11% increased risk of lung cancer for every 100 mg/day increase, and intakes greater than 215 mg/day were specifically linked to a 29% increase in risk for non-small cell lung cancer (52).
To date, most clinical trials have failed to find any beneficial effects of vitamin E supplementation on the risk of various cancers. A randomized, placebo-controlled trial (RCT) in 39,876 women participating in the Women's Health Study found that supplementation with 600 IU (400 mg) of RRR-α-tocopherol every other day for 10 years had no effect on overall cancer incidence, tissue-specific cancer incidence, or cancer-related deaths (37). Yet, the results of a few large randomized controlled trials have suggested that vitamin E supplementation might affect the risk of prostate cancer. The Alpha-Tocopherol, Beta-Carotene cancer (ATBC) prevention study was a four-arm, randomized, double-blind, placebo-controlled trial designed to investigate the effect of α-tocopherol supplementation on lung cancer development in 29,133 male smokers. The study found a 32% reduction in the incidence of prostate cancer in participants given daily supplements of 50 mg of synthetic α-tocopherol (equivalent to 25 mg of RRR-α-tocopherol) alone or in combination with β-carotene compared to those given β-carotene alone or a placebo (53). However, no differences in the incidence of prostate cancer were found between α-tocopherol recipients and nonrecipients during the 18-year post-intervention period (54). In the Physicians’ Health Study II (PHS II), which followed 14,641 healthy men aged 50 years and older, supplementation with 400 IU of synthetic vitamin E (equivalent to 180 mg of RRR-α-tocopherol) every other day for eight years had no effect on the risk of prostate cancer, other site-specific cancers, or total cancer (55). The supplementation of vitamin E (equivalent to 180 mg/day of RRR-α-tocopherol), alone or in combination with selenium, in the multicenter, randomized, placebo-controlled SELECT trial (SELenium and vitamin E Cancer prevention Trial) was halted because there was no evidence of benefit in preventing prostate cancer in 35,533 healthy men aged 50 years and older (56). After a median of seven years’ follow-up, the risk of prostate cancer was found to be significantly increased by 17% in participants supplemented with vitamin E alone during the trial period — but not when vitamin E was combined with selenium — compared to placebo (57). A study of cases versus subcohort individuals drawn from the SELECT study assessed the effect of vitamin E and/or selenium supplementation on prostate cancer risk in relation to the selenium status of participants at baseline (58). Supplemental selenium with or without vitamin E was associated with a significant increase in the risk of advanced prostate cancer in individuals with higher versus lower selenium status. In addition, the risks of total and advanced prostate cancer were significantly elevated with vitamin E supplementation in subjects with low versus high selenium status (58). Recent investigations have suggested that sequence variations (polymorphisms) in vitamin E-related genes and genes coding for antioxidant enzymes, including selenoproteins, might modify the impact of high-dose vitamin E and selenium on the risk of prostate cancer (59-61).
Cataracts
Age-related cataracts appear to be the result of protein oxidation in the lens of the eye; antioxidants like α-tocopherol may protect the lens against oxidative damage from reactive oxygen species. In a recent cross-sectional study, vitamin E concentrations were found to be significantly lower in the lens and blood of subjects with age-related nuclear, but not cortical, cataracts when compared with an age-matched control group (62). However, earlier studies reported higher vitamin E concentrations in the lenses and blood of patients with cataracts (63, 64).
The results of several observational studies that examined the association between vitamin E consumption and the incidence or severity of cataracts are also mixed. Some reported that increased vitamin E intake protected against cataract development, while others found no association (65). Yet, a meta-analysis of eight studies, including 15,021 participants, found a 17% reduction in the risk of age-related cataract in subjects in the highest versus lowest quantile of dietary vitamin E intake (66). A recent prospective cohort study of 31,120 Swedish men followed for a mean of 8.4 years observed a greater risk of developing cataract in occasional and regular users of high-dose (about 100 mg/day) vitamin E supplements only, when compared with non-supplement users (67). However, the use of supplemental high-dose vitamin E with additional supplements or the use of low-dose vitamin E-containing multivitamin supplements was not found to be associated with an elevated cataract risk. A meta-analysis based on data from over 350,000 participants in 10 studies — including the above-cited study by Zheng Selin et al. (67) — found no association between supplemental vitamin E and risk of cataract (66).
In clinical settings, the supplementation of high-dose vitamin E — alone or in addition to other supplements — was found to be safe, yet the benefits regarding cataract risk or progression were limited. An early intervention trial found that a daily supplement of 50 mg of synthetic α-tocopherol (equivalent to 25 mg of RRR-α-tocopherol) did not alter the incidence of cataract surgery in male smokers (68). A randomized, placebo-controlled intervention trial in 4,629 men and women found that a daily antioxidant supplement containing 500 mg of vitamin C, 400 IU of all-rac-α-tocopheryl acetate (equivalent to 180 mg of RRR-α-tocopherol), and 15 mg of β-carotene did not affect development and progression of age-related cataracts over a seven-year period (69). Similarly, a four-armed, randomized, placebo-controlled study of 11,267 men from the Selenium and Vitamin E Cancer prevention trial (SELECT) failed to observe a reduction in cataract incidence with 400 IU/day of supplemental all-rac-α-tocopheryl acetate (180 mg/day of RRR-α-tocopherol), alone or in combination with selenium (200 μg/day), during a mean 5.6 years of follow-up (70). Daily antioxidant supplementation with 500 mg of vitamin C, 400 IU (268 mg) of RRR-α-tocopherol, and 15 mg of β-carotene did not limit the progression of cataract in a five-year intervention trial (71). Another four-year randomized, placebo-controlled trial reported that supplements containing 500 IU/day (335 mg/day) of RRR-α-tocopherol did not reduce the incidence or progression of cataract in older adults (72). Current available data from clinical trials do not support a preventative effect of vitamin E on cataracts.
Disease Treatment
Age-related macular degeneration
A recent pooled analysis of four randomized controlled trials in 62,520 subjects found that supplemental vitamin E or β-carotene did not reduce the risk of developing age-related macular degeneration (AMD), a multifactorial disease affecting the central area of the retina (73). However, a review of currently available data suggested that supplements of antioxidants plus zinc may reduce the progression of AMD and vision loss in affected individuals (74). The main evidence came from the Age-Related Eye Disease Study (AREDS). In this clinical trial, participants with borderline to advanced age-related macular degeneration (AMD) were randomized to receive (1) placebo; (2) antioxidant vitamins (15 mg/day of β-carotene, 500 mg/day of ascorbic acid, and 400 IU/day of all-rac-α-tocopheryl acetate); (3) zinc (80 mg/day) and copper (2 mg/day); or (4) both antioxidant vitamins and zinc and copper (75). The five-year results indicated that the risk of developing advanced AMD was significantly reduced in those taking zinc with or without antioxidant vitamins. Antioxidant vitamins alone failed to prevent the progression to advanced AMD, even in individuals at higher risk. It was concluded from this study that a combination of antioxidant vitamins and minerals may benefit people with intermediate AMD or advanced AMD in one eye (74, 76).
Type 2 diabetes mellitus
Oxidative stress contributes to the progression of type 2 diabetes mellitus and causes damage to many organs and tissues, including the pancreas, brain, eyes, peripheral nerves, and kidneys. Evidence from animal studies suggests that vitamin E supplementation could mitigate the role of oxidative damage in the occurrence of diabetes complications (reviewed in 77). In the Alpha-Tocopherol Beta-Carotene cancer prevention (ATBC) trial in male smokers, supplementation with 50 mg/day of synthetic α-tocopherol (25 mg/day of RRR-α-tocopherol) had no effect on the risk of incident type 2 diabetes mellitus during the 19-year post-intervention follow-up. Likewise, supplemental vitamin E intake during the trial made no difference on the incidence of macrovascular complications or mortality in participants with established type 2 diabetes (78). In addition, a meta-analysis of 14 heterogeneous randomized controlled trials, including 714 type 2 diabetic individuals, found that supplementation with vitamin E (200-1,800 IU/day for 6-27 weeks) had no effect on markers of glycemic control, including glycated hemoglobin A1c (HbA1c) level and measures of fasting glucose and fasting insulin concentrations (79). Further subgroup analyses indicated that higher doses of vitamin E (>400 IU/day) supplemented for longer periods (>12 weeks) significantly reduced HbA1c level and fasting insulin concentration, suggesting that vitamin E could possibly enhance insulin action and glucose disposal in type 2 diabetic individuals (79). Another recent meta-analysis of randomized controlled trials found that endothelial function in normal-weight and overweight — but not obese — patients with type 2 diabetes was significantly improved by supplementation with vitamin E and/or vitamin C (80). Although there is reason to suspect that vitamin E supplementation may have utility in the management of type 2 diabetes, evidence for benefit from large, well-controlled clinical trials is still lacking.
Fatty liver diseases
The increasing incidence of nonalcoholic fatty liver disease (NAFLD) in children and adults in industrialized countries is mainly attributed to the ongoing epidemic of obesity and type 2 diabetes mellitus. NAFLD results from the abnormal accumulation of fat (steatosis) in the liver in the absence of heavy alcohol consumption. Although the condition is considered to be largely benign, NAFLD can progress to a more severe disease called nonalcoholic steatohepatitis (NASH) with increased risks of cirrhosis, hepatocellular carcinoma (liver cancer), and cardiovascular disease (81). Oxidative stress is thought to be one of the possible mechanisms responsible for prompting inflammatory processes that can lead to the progression of NAFLD to NASH.
There is currently no established treatment for NAFLD and NASH other than interventions that encourage lifestyle changes and the use of medicines to control or treat metabolic disorders (82). In the multicenter PIVENS (PIoglitazone versus Vitamin E versus placebo for the treatment of Nonalcoholic Steatohepatitis) trial, 247 nondiabetic subjects with NASH were randomized to receive 30 mg/day of pioglitazone (an insulin-sensitizing drug), 800 IU/day (536 mg/day) of RRR-α-tocopherol, or a placebo for 96 weeks (83). Only vitamin E supplementation significantly increased the overall rate of improvement in histological abnormalities that characterize NASH on liver biopsies (i.e., hepatocellular ballooning, steatosis, and lobular inflammation) (84). Both active treatments improved some markers of liver function (i.e., alanine aminotransferase and aspartate aminotransferase) (84). Yet, results from another two-year, randomized controlled trial — called TONIC for Treatment Of Nonalcoholic fatty liver disease In Children — in 173 children (ages, 8-17 years) with NAFLD failed to observe any significant reduction in blood concentrations of alanine and aspartate aminotransferases either with supplemental vitamin E (536 mg/day of RRR-α-tocopherol) or with metformin (an anti-diabetic drug; 1,000 mg/day) compared to placebo (85). However, vitamin E supplementation significantly improved the overall disease activity score — used to quantify the severity of the disease. In addition, a recent meta-analysis of another six trials found that vitamin E significantly lowered circulating aminotransferase concentrations in NAFLD and NASH patients, suggesting liver function improvements (86). Finally, in a small nonrandomized, unblinded, controlled study in 42 obese children (mean age, 8 years) with NAFLD, lifestyle recommendations combined with 600 mg/day of supplemental RRR-α-tocopheryl acetate for six months reduced markers of oxidative stress and liver dysfunction and improved insulin sensitivity and the profile of lipid in the blood, when compared to baseline. No such changes in markers of oxidative stress, liver function, and glucose utilization were reported in the lifestyle intervention only group (87). Further randomized and well-controlled studies are needed to confirm these preliminary findings.
Cognitive deterioration and Alzheimer's disease
Mitochondrial dysfunction and oxidative stress are thought to contribute to the onset and/or progression of several neurodegenerative diseases, especially Alzheimer's disease (AD) (88). The progressive degeneration of neuronal cells that accompanies the decline of memory and other cognitive functions in subjects with Alzheimer’s disease is associated with an intracellular aggregation of Tau fibrils, an extraneuronal accumulation of β-amyloid peptides into senile plaques, and an oxidation-reduction (redox) imbalance of complex etiology. In the brain of patients with mild cognitive impairment (MCI) and those with AD, the level of markers of oxidative damage to DNA, proteins, and lipids is increased, while the expression and activities of glutathione and antioxidant enzymes are reduced (reviewed in 88). In addition, a recent meta-analysis reported that circulating concentrations of vitamins, including vitamin A, vitamin C, and vitamin E, were significantly lower in AD patients than in cognitively healthy individuals (89). Other studies have documented low concentrations of vitamin E in cerebrospinal fluid of cognitively impaired patients (reviewed in 90).
Because a reduction in oxidative stress may help maintain cognitive status and/or prevent deterioration, the effects of vitamin E supplementation have been assessed in a few intervention studies. An early multicenter, randomized, placebo-controlled study in individuals with AD of moderate severity found that supplementation with 2,000 IU/day of all-rac-α-tocopherol (equivalent to 900 mg/day of RRR-α-tocopherol) for two years significantly delayed cognitive decline, slowed disease progression, and increased median survival (91). However, a placebo-controlled trial in 769 patients with MCI found that the same dosage of vitamin E did not affect the probability of progression from MCI to AD over a three-year period (92). In another double-blind, placebo-controlled trial, an improvement in cognitive performance — measured by the Mini Mental State Examination (MMSE) scoring system — was reported in AD patients randomized to receive 800 IU/day of all-rac-α-tocopherol (360 mg/day of RRR-α-tocopherol) for six months only when the treatment effectively reduced oxidative stress (as assessed by the measure of total glutathione and markers of lipid peroxidation in the blood) (93). Conversely, a failure to reduce oxidative stress resulted in supplemental vitamin E being more detrimental to the cognitive function of AD patients than placebo. In the most recent multicenter, randomized, double-blind, placebo-controlled study, supplemental vitamin E (2,000 IU/day; form of vitamin E not mentioned in the publication) for over two years significantly delayed functional decline — determined by the (in)ability to perform basic activities of daily living — and reduced the annual mortality rate in mild and moderate AD patients (94). Yet, vitamin E failed to affect cognitive performance measured with MMSE scores and other cognitive ability tests.
While there is currently little evidence to suggest that long-term supplementation of vitamin E provides any cognitive benefits in healthy older adults (95), additional research needs to confirm whether vitamin E supplementation could benefit the management of patients with mild-to-moderate cognitive impairments.
Sources
Food sources
Major sources of α-tocopherol in the American diet include vegetable oils (olive, sunflower, and safflower oils), nuts, whole grains, and green leafy vegetables. All eight forms of vitamin E (α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols) occur naturally in mostly plant-based foods but in varying amounts. Table 2 lists the content of α-tocopherol and γ-tocopherol (in milligrams) in some rich sources of vitamin E. For more information on the vitamin E content of foods, search USDA's FoodData Central.
In the US, the average intake of α-tocopherol from food (including enriched and fortified sources) for adults (≥19 years of age) is 7.2 mg/day (25); this level is well below the RDA of 15 mg/day of α-tocopherol (see Table 1). While it appears feasible for individuals to meet the RDA from food only, Americans would have to depart from their current dietary practices and include greater intakes of nuts, seeds, fruit, and vegetables without increasing fat intake above recommended levels (96).
Supplements
RRR-α-tocopherol is the only stereoisomeric form of α-tocopherol found in unfortified foods. The same is not always true for nutritional supplements. Vitamin E supplements generally contain 100 IU to 1,000 IU of α-tocopherol. Supplements made from entirely natural sources contain only RRR-α-tocopherol (wrongly labeled d-α-tocopherol). RRR-α-tocopherol is the most bioavailable form of α-tocopherol in the body. Synthetic α-tocopherol, which is often found in fortified food and nutritional supplements and usually labeled all-rac-α-tocopherol or dl-α-tocopherol, include all eight possible stereoisomers of α-tocopherol (see Function). Because half of the isomers present as a mixture in synthetic α-tocopherol are not usable by the body, synthetic α-tocopherol is less bioavailable than natural α-tocopherol (see Figure 1). To calculate the amount (in milligrams) of α-tocopherol bioavailable in a supplement, the conversion factors are as follows:
-
Natural vitamin E (RRR-α-tocopherol) containing supplements:
IU of RRR-α-tocopherol x 0.67 = mg of RRR-α-tocopherol
Example: 100 IU of natural vitamin E provides 67 mg of RRR-α-tocopherol
-
Synthetic vitamin E (all-rac-α-tocopherol) containing supplements:
IU of all-rac-α-tocopherol x 0.45 = mg of RRR-α-tocopherol
Example: 100 IU of synthetic vitamin E provides 45 mg of 2R-α-tocopherol
In addition, vitamin E-fortified foods often contain synthetic α-tocopherol, and amounts of vitamin E are provided as a percentage of the daily value (DV) of 30 IU (approximately 20 mg of RRR-α-tocopherol).
α-Tocopheryl esters
α-Tocopheryl succinate and α-tocopheryl acetate are the main esterified forms of vitamin E in nutritional supplements. Tocopherol esters are more resistant to oxidation during storage than unesterified tocopherols (1). When taken orally, the succinate or acetate moieties are removed from α-tocopherol in the intestine. The bioavailability of α-tocopherol from α-tocopheryl succinate and α-tocopheryl acetate is equivalent to that of free α-tocopherol (97). Hence, the conversion factors used to determine the amount of bioavailable α-tocopherol provided by α-tocopheryl succinate and α-tocopheryl acetate are the same as those used for α-tocopherol (see above) (2). Cell culture studies indicated that the vitamin E ester, α-tocopheryl succinate, could inhibit proliferation and induce apoptosis in a number of cancer cell lines (12). Limited data from animal models of cancer found that α-tocopheryl succinate administered by injection inhibited tumor growth (98). There is currently no evidence in humans that taking oral α-tocopheryl succinate supplements delivers α-tocopheryl succinate to tissues. Of note, current research investigates nanomedicines to increase α-tocopheryl succinate bioavailability before exploring putative benefits in clinical settings (98).
α-Tocopheryl nicotinate is another α-tocopherol ester formed from synthetic α-tocopherol and nicotinic acid (niacin). While α-tocopheryl nicotinate can be prescribed as a lipid-lowering agent in Europe and Japan, it is marketed as a supplement only in the US (99).
α-Tocopheryl phosphates (Ester-E®)
There is currently no published evidence that supplements containing α-tocopheryl phosphates are more efficiently absorbed or have greater bioavailability in humans than those containing α-tocopherol (99).
Other supplemental forms
Supplements containing γ-tocopherol, mixed tocopherols, or tocotrienols are also commercially available (99). The amounts of α- and γ-tocopherol in mixed tocopherol supplements vary, so it is important to read the label to determine the amount of each tocopherol form present in a capsule.
Safety
Toxicity
Few side effects have been noted in adults taking supplements of less than 2,000 mg of α-tocopherol daily (either natural or synthetic vitamin E). However, most studies assessing safety issues or toxicity of α-tocopherol supplementation have lasted only a few weeks to a few months, and side effects associated with long-term α-tocopherol supplementation have not been adequately studied. The most worrisome possibility is that of impaired blood clotting, which increases the likelihood of hemorrhage in some individuals. A meta-analysis of randomized controlled trials found that daily vitamin E supplementation — equivalent to 25 to 536 mg/day of RRR-α-tocopherol — for several years resulted in a significant, 10% reduction in the risk of ischemic stroke (five trials, 91,393 participants) and a nonsignificant trend towards an increased risk of hemorrhagic stroke (five trials, 100,748 participants) (100).
A tolerable upper intake level (UL) for any form of supplemental α-tocopherol (all possible stereoisomers) has been established by the Food and Nutrition Board of the Institute of Medicine to avoid the potential risk of bleeding (Table 3). Specifically, the UL of 1,000 mg/day of α-tocopherol in any supplemental form (equivalent to 1,500 IU/day of RRR-α-tocopherol or 1,100 IU/day of all-rac-α-tocopherol) corresponds to the highest dose unlikely to result in hemorrhage in almost all adults (2). Although only certain isomers of α-tocopherol are retained in the circulation, all forms are absorbed and metabolized by the liver. Hence, the rationale for a UL that refers to all stereoisomers of α-tocopherol is based on the fact that any form of α-tocopherol (natural or synthetic) can be absorbed and thus be potentially harmful.
| Age Group | mg/day# |
|---|---|
| Infants 0-12 months | Not possible to establish## |
| Children 1-3 years | 200 |
| Children 4-8 years | 300 |
| Children 9-13 years | 600 |
| Adolescents 14-18 years | 800 |
| Adults 19 years and older | 1,000 |
|
*The UL for α-tocopherol applies to all stereoisomers of α-tocopherol (natural and synthetic) found in supplements and fortified food. |
|
Some physicians recommend discontinuing high-dose vitamin E supplementation two to four weeks before elective surgery — including dental procedures — to decrease the risk of hemorrhage (99).
Because dietary vitamin E is essential to prevent vitamin E deficiency in the newborn, vitamin E must be supplied in parenteral nutrition solutions in infants who cannot be given enteral feeding, such as prematurely born infants. Yet, preterm infants appear to be especially vulnerable to adverse effects of α-tocopherol supplementation, and supplemental vitamin E should be administered only under controlled supervision by a pediatrician (101).
Finally, the results of only one randomized controlled trial in 601 patients with common forms of retinitis pigmentosa (RP) indicated that supplementation with 400 IU/day of synthetic vitamin E (equivalent to 180 mg/day of RRR-α-tocopherol) modestly but significantly accelerated the loss of retinal function compared to placebo (22). Patients with common forms of RP should therefore avoid taking high-dose vitamin E supplements if they are not deficient in vitamin E (see Deficiency).
Does vitamin E supplementation increase the risk of all-cause mortality?
A prospective observational study in over 4,000 participants of the Framingham Heart Study and the Framingham Offspring Study — with or without preexisting cardiovascular disease — found no statistically significant association between vitamin E supplement intake and cardiovascular or all-cause mortality after a 10-year follow-up period (102). However, in addition to reports of increased risk of hemorrhage and heart failure with supplemental vitamin E in several randomized controlled studies (see Cardiovascular disease), a meta-analysis by Miller et al. (103) suggested an increased risk of death with the use of large doses of vitamin E, yet lower than the UL. Specifically, this meta-analysis combined the results of 19 clinical trials of vitamin E supplementation that mostly focused on secondary prevention and, as such, included subjects with pre-existing conditions including heart disease, end-stage renal failure, and Alzheimer's disease. The study found that daily supplementation with at least 400 IU of synthetic vitamin E (equivalent to 180 mg of RRR-α-tocopherol) resulted in a 4% increase in risk of death from any cause compared to placebo (103). However, further dose-response analysis and adjustment for intake of other vitamin and mineral supplements indicated that all-cause mortality risk was significantly increased by 7% only at a dose of 2,000 IU/day, which is notably higher than the UL for adults (1,100 IU/day of synthetic tocopherol or 1,500 IU/day of natural tocopherol; see Table 3). Additionally, a more recent meta-analysis of 46 randomized trials, including 171,244 participants, found that supplemental vitamin E, singly or in combination with other antioxidants, did not significantly alter the risk of all-cause mortality (104). At present, there is no convincing evidence that vitamin E supplementation below the UL increases the risk of death from cardiovascular disease or other causes, especially in generally healthy subjects. Yet, individuals with pre-existing conditions may be at increased risk of serious adverse effects (including death) if one considers the possibility that large doses of supplemental vitamin E might interfere with medications, and possibly lower their efficacy or increase their toxicity (1).
Nutrient interactions
Large doses of vitamin E may inhibit vitamin K-dependent carboxylase activity and interfere with the coagulation cascade (see the article on Vitamin K) (19). Hence, the use of vitamin E supplements may increase the risk of bleeding in individuals taking anticoagulant drugs (blood thinners), such as heparin and the vitamin K antagonist, warfarin (Coumadin); antiplatelet drugs, such as clopidogrel (Plavix), ticlopidine (Ticlid), tirofiban (Aggrastat), and dipyridamole (Aggrenox); and non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin, ibuprofen, and others. In addition, individuals who may be vitamin K deficient due to liver failure, those with a propensity to bleed (e.g., bleeding peptic ulcers), and those with inherited bleeding disorders (e.g., hemophilia) or a history of hemorrhagic stroke, should not take α-tocopherol supplements without close medical supervision because of the increased risk of hemorrhage (20, 99). Finally, it cannot be excluded that vitamin E would potentiate the antithrombotic activity of supplemental fish oils and herbal products, such as garlic, curcumin, or Ginkgo biloba (99).
Drug interactions
A number of cholesterol-lowering medications (like cholestyramine and colestipol), as well as orlistat, sucralfate, mineral oil, and the fat substitute, olestra, which interfere with fat absorption, may theoretically decrease the absorption of fat-soluble vitamins, including vitamin E. The anticonvulsant drugs phenobarbital, phenytoin (Dilantin), and carbamazepine (Tegretol), may also lower plasma vitamin E concentrations in individuals with epilepsy (105).
Antioxidants and statins (3-hydroxy-3-methylglutary-coenzyme A reductase inhibitors)
A three-year randomized controlled trial in 160 patients with coronary heart disease (CHD) and low high-density-lipoprotein (HDL) levels found that a combination of simvastatin (Zocor) and niacin increased the HDL2 subfraction level (considered the most cardioprotective), inhibited the progression of coronary artery stenosis (narrowing), and decreased the frequency of cardiovascular events, such as myocardial infarction and stroke (106). Surprisingly, when an antioxidant combination of 1,000 mg of vitamin C, 800 IU (536 mg) of RRR-α-tocopherol, 100 μg of selenium, and 25 mg of β-carotene daily, was taken with the simvastatin-niacin combination, the protective effects were diminished. However, in a much larger randomized controlled trial of simvastatin and an antioxidant combination of 600 mg of all-rac-α-tocopherol (297 mg of RRR-α-tocopherol), 250 mg of vitamin C, and 20 mg of β-carotene daily in more than 20,000 men and women with CHD or diabetes, the antioxidant combination did not adversely affect the cardioprotective effects of simvastatin therapy over a five-year period (107). These contradictory findings indicate that further research is needed on potential interactions between antioxidant supplementation and cholesterol-lowering agents like statins.
Linus Pauling Institute Recommendation
The Recommended Dietary Allowance (RDA) for vitamin E for adult men and women is 15 mg (22.5 IU) per day. Notably, more than 90% of individuals two years of age and older in the US do not meet the daily requirement for vitamin E from food sources alone. Therefore, LPI recommends that generally healthy adults (aged 19 years and older) take a daily multivitamin/mineral (MVM) supplement, which usually contains 30 IU of synthetic vitamin E — equivalent to 13.5 mg of RRR-α-tocopherol and 90% of the RDA.
Older adults (>50 years)
The Linus Pauling Institute’s recommendation to take a daily multivitamin/mineral (MVM) supplement containing vitamin E is also appropriate for generally healthy older adults. MVMs typically contain 30 IU of synthetic vitamin E, covering 90% of the RDA.
Authors and Reviewers
Originally written in 2000 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2004 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in June 2008 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in May 2015 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in October 2015 by:
Maret G. Traber, Ph.D.
Helen P. Rumbel Professor for Micronutrient Research, Linus Pauling Institute
Professor, College of Public Health and Human Sciences
Oregon State University
Copyright 2000-2024 Linus Pauling Institute
References
1. Traber MG. Vitamin E. In: Erdman JWJ, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, D.C.: Wiley-Blackwell; 2012:214-229.
2. Food and Nutrition Board, Institute of Medicine. Vitamin E. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C.: National Academy Press; 2000:186-283. (National Academy Press)
3. Trpkovic A, Resanovic I, Stanimirovic J, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2014:1-16. (PubMed)
4. Davis S, Davis BM, Richens JL, et al. α-Tocopherols modify the membrane dipole potential leading to modulation of ligand binding by P-glycoprotein. J Lipid Res. 2015;56(8):1543-1550. (PubMed)
5. Marko MG, Ahmed T, Bunnell SC, et al. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol. 2007;178(3):1443-1449. (PubMed)
6. Molano A, Meydani SN. Vitamin E, signalosomes and gene expression in T cells. Mol Aspects Med. 2012;33(1):55-62. (PubMed)
7. Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76-90. (PubMed)
8. Jiang Q, Christen S, Shigenaga MK, Ames BN. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74(6):714-722. (PubMed)
9. Mah E, Pei R, Guo Y, et al. γ-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic Biol Med. 2013;65:1291-1299. (PubMed)
10. Mah E, Pei R, Guo Y, et al. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2α. Exp Biol Med (Maywood). 2015;240(4):527-533. (PubMed)
11. Ahsan H, Ahad A, Iqbal J, Siddiqui WA. Pharmacological potential of tocotrienols: a review. Nutr Metab (Lond). 2014;11(1):52. (PubMed)
12. Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123(4):739-752. (PubMed)
13. Fu JY, Che HL, Tan DM, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutr Metab (Lond). 2014;11(1):5. (PubMed)
14. Bruno RS, Leonard SW, Park SI, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am J Clin Nutr. 2006;83(2):299-304. (PubMed)
15. Leonard SW, Good CK, Gugger ET, Traber MG. Vitamin E bioavailability from fortified breakfast cereal is greater than that from encapsulated supplements. Am J Clin Nutr. 2004;79(1):86-92. (PubMed)
16. Traber MG, Leonard SW, Bobe G, et al. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr. 2015;101(4):752-759. (PubMed)
17. Leonard SW, Bruno RS, Ramakrishnan R, Bray T, Traber MG. Cigarette smoking increases human vitamin E requirements as estimated by plasma deuterium-labeled CEHC. Ann N Y Acad Sci. 2004;1031:357-360. (PubMed)
18. Bruno RS, Leonard SW, Atkinson J, et al. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40(4):689-697. (PubMed)
19. Booth SL, Golly I, Sacheck JM, et al. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004;80(1):143-148. (PubMed)
20. Pastori D, Carnevale R, Cangemi R, et al. Vitamin E serum levels and bleeding risk in patients receiving oral anticoagulant therapy: a retrospective cohort study. J Am Heart Assoc. 2013;2(6):e000364. (PubMed)
21. Traber MG. Vitamin E. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2014:293-304.
22. Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111(6):761-772. (PubMed)
23. Iwasa K, Shima K, Komai K, Nishida Y, Yokota T, Yamada M. Retinitis pigmentosa and macular degeneration in a patient with ataxia with isolated vitamin E deficiency with a novel c.717 del C mutation in the TTPA gene. J Neurol Sci. 2014;345(1-2):228-230. (PubMed)
24. Ford ES, Sowell A. Serum α-tocopherol status in the United States population: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 1999;150(3):290-300. (PubMed)
25. Fulgoni VL, 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011;141(10):1847-1854. (PubMed)
26. Shamim AA, Schulze K, Merrill RD, et al. First-trimester plasma tocopherols are associated with risk of miscarriage in rural Bangladesh. Am J Clin Nutr. 2015;101(2):294-301. (PubMed)
27. Wright ME, Lawson KA, Weinstein SJ, et al. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006;84(5):1200-1207. (PubMed)
28. Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283-289. (PubMed)
29. De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res. 2008;42(3):272-280. (PubMed)
30. Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277(17):1380-1386. (PubMed)
31. Pallast EG, Schouten EG, de Waart FG, et al. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr. 1999;69(6):1273-1281. (PubMed)
32. Meydani SN, Leka LS, Fine BC, et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292(7):828-836. (PubMed)
33. Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139(12):1180-1189. (PubMed)
34. Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334(18):1156-1162. (PubMed)
35. Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328(20):1450-1456. (PubMed)
36. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328(20):1444-1449. (PubMed)
37. Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56-65. (PubMed)
38. Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study. Circulation. 2007;116(13):1497-1503. (PubMed)
39. Violi F, Pignatelli P. Letter by Violi and Pignatelli regarding article, "Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study". Circulation. 2008;117(15):e312; author reply e313. (PubMed)
40. Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123-2133. (PubMed)
41. Riccioni G, D'Orazio N, Palumbo N, et al. Relationship between plasma antioxidant concentrations and carotid intima-media thickness: the Asymptomatic Carotid Atherosclerotic Disease In Manfredonia Study. Eur J Cardiovasc Prev Rehabil. 2009;16(3):351-357. (PubMed)
42. Riccioni G, Bazzano LA. Antioxidant plasma concentration and supplementation in carotid intima media thickness. Expert Rev Cardiovasc Ther. 2008;6(5):723-729. (PubMed)
43. Azen SP, Qian D, Mack WJ, et al. Effect of supplementary antioxidant vitamin intake on carotid arterial wall intima-media thickness in a controlled clinical trial of cholesterol lowering. Circulation. 1996;94(10):2369-2372. (PubMed)
44. Joris PJ, Mensink RP. Effects of supplementation with the fat-soluble vitamins E and D on fasting flow-mediated vasodilation in adults: a meta-analysis of randomized controlled trials. Nutrients. 2015;7(3):1728-1743. (PubMed)
45. Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet. 1996;347(9004):781-786. (PubMed)
46. Boaz M, Smetana S, Weinstein T, et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356(9237):1213-1218. (PubMed)
47. Milman U, Blum S, Shapira C, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28(2):341-347. (PubMed)
48. Leppala JM, Virtamo J, Fogelholm R, et al. Controlled trial of α-tocopherol and β-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20(1):230-235. (PubMed)
49. Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338-1347. (PubMed)
50. Marchioli R, Levantesi G, Macchia A, et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown). 2006;7(5):347-350. (PubMed)
51. Dizdaroglu M. Oxidatively induced DNA damage: mechanisms, repair and disease. Cancer Lett. 2012;327(1-2):26-47. (PubMed)
52. Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am J Respir Crit Care Med. 2008;177(5):524-530. (PubMed)
53. Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with α-tocopherol and β-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90(6):440-446. (PubMed)
54. Virtamo J, Taylor PR, Kontto J, et al. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int J Cancer. 2014;135(1):178-185. (PubMed)
55. Wang L, Sesso HD, Glynn RJ, et al. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr. 2014;100(3):915-923. (PubMed)
56. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39-51. (PubMed)
57. Klein EA, Thompson IM, Jr., Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549-1556. (PubMed)
58. Kristal AR, Darke AK, Morris JS, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106(3):djt456. (PubMed)
59. Cheng TY, Barnett MJ, Kristal AR, et al. Genetic variation in myeloperoxidase modifies the association of serum α-tocopherol with aggressive prostate cancer among current smokers. J Nutr. 2011;141(9):1731-1737. (PubMed)
60. Gerstenberger JP, Bauer SR, Van Blarigan EL, et al. Selenoprotein and antioxidant genes and the risk of high-grade prostate cancer and prostate cancer recurrence. Prostate. 2015;75(1):60-69. (PubMed)
61. Major JM, Yu K, Weinstein SJ, et al. Genetic variants reflecting higher vitamin e status in men are associated with reduced risk of prostate cancer. J Nutr. 2014;144(5):729-733. (PubMed)
62. Katta AV, Katkam RV, Geetha H. Lipid peroxidation and the total antioxidant status in the pathogenesis of age related and diabetic cataracts: a study on the lens and blood. J Clin Diagn Res. 2013;7(6):978-981. (PubMed)
63. Ferrigno L, Aldigeri R, Rosmini F, Sperduto RD, Maraini G, Italian-American Cataract Study G. Associations between plasma levels of vitamins and cataract in the Italian-American Clinical Trial of Nutritional Supplements and Age-Related Cataract (CTNS): CTNS Report #2. Ophthalmic Epidemiol. 2005;12(2):71-80. (PubMed)
64. Krepler K, Schmid R. α-Tocopherol in plasma, red blood cells and lenses with and without cataract. Am J Ophthalmol. 2005;139(2):266-270. (PubMed)
65. West AL, Oren GA, Moroi SE. Evidence for the use of nutritional supplements and herbal medicines in common eye diseases. Am J Ophthalmol. 2006;141(1):157-166. (PubMed)
66. Zhang Y, Jiang W, Xie Z, Wu W, Zhang D. Vitamin E and risk of age-related cataract: a meta-analysis. Public Health Nutr. 2015:1-11. (PubMed)
67. Zheng Selin J, Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. High-dose supplements of vitamins C and E, low-dose multivitamins, and the risk of age-related cataract: a population-based prospective cohort study of men. Am J Epidemiol. 2013;177(6):548-555. (PubMed)
68. Teikari JM, Rautalahti M, Haukka J, et al. Incidence of cataract operations in Finnish male smokers unaffected by α-tocopherol or β-carotene supplements. J Epidemiol Community Health. 1998;52(7):468-472. (PubMed)
69. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and β-carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439-1452. (PubMed)
70. Christen WG, Glynn RJ, Gaziano JM, et al. Age-related cataract in men in the selenium and vitamin E cancer prevention trial eye endpoints study: a randomized clinical trial. JAMA Ophthalmol. 2015;133(1):17-24. (PubMed)
71. Gritz DC, Srinivasan M, Smith SD, et al. The Antioxidants in Prevention of Cataracts Study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90(7):847-851. (PubMed)
72. McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004;111(1):75-84. (PubMed)
73. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. 2012;6:CD000253. (PubMed)
74. Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2012;11:CD000254. (PubMed)
75. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, β-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436. (PubMed)
76. Hobbs RP, Bernstein PS. Nutrient Supplementation for Age-related Macular Degeneration, Cataract, and Dry Eye. J Ophthalmic Vis Res. 2014;9(4):487-493. (PubMed)
77. Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131(4):276-286. (PubMed)
78. Kataja-Tuomola MK, Kontto JP, Mannisto S, Albanes D, Virtamo JR. Effect of α-tocopherol and β-carotene supplementation on macrovascular complications and total mortality from diabetes: results of the ATBC Study. Ann Med. 2010;42(3):178-186. (PubMed)
79. Xu R, Zhang S, Tao A, Chen G, Zhang M. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e95008. (PubMed)
80. Montero D, Walther G, Stehouwer CD, Houben AJ, Beckman JA, Vinet A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014;15(2):107-116. (PubMed)
81. Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5(3):211-218. (PubMed)
82. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55(4):885-904. (PubMed)
83. Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials. 2009;30(1):88-96. (PubMed)
84. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685. (PubMed)
85. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659-1668. (PubMed)
86. Ji HF. Vitamin E therapy on aminotransferase levels in NAFLD/NASH patients. Nutrition. 2015;31(6):899. (PubMed)
87. D'Adamo E, Marcovecchio ML, Giannini C, et al. Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic Res. 2013;47(3):146-153. (PubMed)
88. Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013;2013:316523. (PubMed)
89. Lopes da Silva S, Vellas B, Elemans S, et al. Plasma nutrient status of patients with Alzheimer's disease: Systematic review and meta-analysis. Alzheimers Dement. 2014;10(4):485-502. (PubMed)
90. Kontush K, Schekatolina S. Vitamin E in neurodegenerative disorders: Alzheimer's disease. Ann N Y Acad Sci. 2004;1031:249-262. (PubMed)
91. Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336(17):1216-1222. (PubMed)
92. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379-2388. (PubMed)
93. Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17(1):143-149. (PubMed)
94. Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33-44. (PubMed)
95. Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166(22):2462-2468. (PubMed)
96. Gao X, Wilde PE, Lichtenstein AH, Bermudez OI, Tucker KL. The maximal amount of dietary α-tocopherol intake in U.S. adults (NHANES 2001-2002). J Nutr. 2006;136(4):1021-1026. (PubMed)
97. Cheeseman KH, Holley AE, Kelly FJ, Wasil M, Hughes L, Burton G. Biokinetics in humans of RRR-α-tocopherol: the free phenol, acetate ester, and succinate ester forms of vitamin E. Free Radic Biol Med. 1995;19(5):591-598. (PubMed)
98. Duhem N, Danhier F, Preat V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J Control Release. 2014;182:33-44. (PubMed)
99. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. 2nd edition ed: Thomson Reuters; 2008.
100. Schurks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. (PubMed)
101. Brion LP, Bell EF, Raghuveer TS, Soghier L. What is the appropriate intravenous dose of vitamin E for very-low-birth-weight infants? J Perinatol. 2004;24(4):205-207. (PubMed)
102. Dietrich M, Jacques PF, Pencina MJ, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: Does the underlying health status play a role? Atherosclerosis. 2009;205(2):549-553. (PubMed)
103. Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37-46. (PubMed)
104. Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with β-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. 2013;8(9):e74558. (PubMed)
105. https://naturalmedicines.therapeuticresearch.com/. Vitamin E professional monograph. Natural Medicines; 2014 Copyright.
106. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583-1592. (PubMed)
107. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23-33. (PubMed)