Isothiocyanates
Contents
Summary
- Isothiocyanates are derived from the hydrolysis of glucosinolates — sulfur-containing compounds found in cruciferous vegetables. (More information)
- Each glucosinolate forms a different isothiocyanate when hydrolyzed. For example, broccoli is a good source of glucoraphanin, the glucosinolate precursor of sulforaphane, and sinigrin, the glucosinolate precursor of allyl isothiocyanate. (More information)
- Absorbed isothiocyanates are rapidly conjugated to glutathione in the liver, and then sequentially metabolized in the mercapturic acid pathway, before being excreted in the urine. (More information)
- Isothiocyanates may modulate the expression and activity of biotransformation enzymes that are involved in the metabolism and elimination of xenobiotics (e.g., carcinogens) from the body. In cultured cells and animal models, isothiocyanates also exhibited antioxidant and anti-inflammatory activities and interfered with numerous cancer-related targets and pathways. (More information)
- Although high intakes of cruciferous vegetables have been associated with a lower risk for cancer, there is insufficient evidence that exposure to isothiocyanates through cruciferous vegetable consumption decreases cancer risk. (More information)
- Glucosinolates are present in relatively high concentrations in cruciferous vegetables, but the amounts of isothiocyanates formed from glucosinolates in foods are variable and depend partly on food processing and preparation. (More information)
Introduction
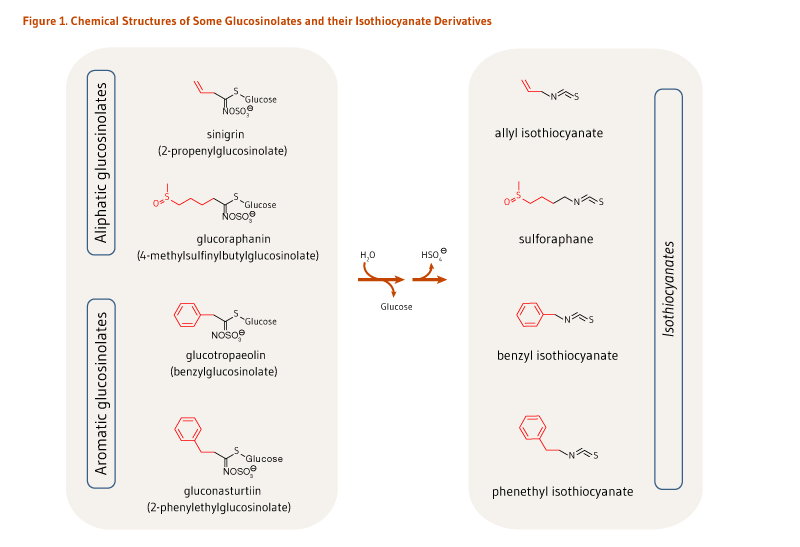
Cruciferous vegetables, such as broccoli, cabbage, and kale, are rich sources of sulfur-containing compounds called glucosinolates (see the article on Cruciferous Vegetables). Isothiocyanates are biologically active hydrolysis (breakdown) products of glucosinolates. Cruciferous vegetables contain a variety of glucosinolates, each of which forms a different isothiocyanate when hydrolyzed (Figure 1) (1). For example, broccoli is a good source of glucoraphanin, the glucosinolate precursor of sulforaphane, and sinigrin, the glucosinolate precursor of allyl isothiocyanate (AITC) (see Food sources) (2). Watercress is a rich source of gluconasturtiin, the precursor of phenethyl isothiocyanate (PEITC), while garden cress is rich in glucotropaeolin, the precursor of benzyl isothiocyanate (BITC) (see Food sources). At present, scientists are interested in the cancer-preventive activities of vegetables that are rich in glucosinolates (see the article on Cruciferous Vegetables), as well as individual isothiocyanates (3).
Metabolism and Bioavailability
Metabolism
The hydrolysis of glucosinolates, which is catalyzed by a class of enzymes called myrosinases (β-thioglucosidases), leads to the formation of breakdown compounds, such as thiocyanates, isothiocyanates, indoles, oxazolidine-2-thiones (e.g., goitrin), epithionitrile, and nitrile (see the article on Cruciferous Vegetables). In intact plant cells, myrosinase is physically separated from glucosinolates. Yet, when plant cells are damaged, myrosinase is released and comes in contact with glucosinolates, catalyzing their conversion into highly reactive metabolites that impart a pungent aroma and spicy (some say bitter) taste. Likewise, when raw cruciferous vegetables are chopped during the food preparation process, glucosinolates are rapidly hydrolyzed by myrosinase, generating metabolites that are then absorbed in the proximal intestine. In contrast, cooking cruciferous vegetables before consumption inactivates myrosinase, thus preventing the breakdown of glucosinolates. However, lightly cooking (i.e., light steam for <5 minutes) will preserve some of the myrosinase and allow for isothiocyanate conversion. A small fraction of intact glucosinolates may be absorbed in the small intestine, but a large proportion reaches the colon (4). In the colon, myrosinase produced by the microbiota can catalyze the generation of a wide range of metabolites from glucosinolates, depending on the pH and the presence of cofactors (4, 5).
The hydrolysis of glucosinolates at neutral pH results in the formation of unique isothiocyanates (Figure 1). For example, sinigrin, glucoraphanin, glucotropaeolin, and gluconasturtiin are the glucosinolate precursors of AITC, sulforaphane, BITC, and PEITC, respectively (Figure 1). Once absorbed, glucosinolate-derived isothiocyanates (like sulforaphane) are promptly conjugated to glutathione by a class of phase II detoxification enzymes known as glutathione S-transferases (GSTs) in the liver, and then sequentially metabolized in the mercapturic acid pathway (Figure 2). This mechanism is meant to increase the solubility of isothiocyanates, thereby promoting a rapid excretion in the urine. Using sulforaphane as the model isothiocyanate, it has indeed been established that its metabolites — sulforaphane-glutathione, sulforaphane-cysteine-glycine, sulforaphane-cysteine, and sulforaphane N-acetylcysteine — collectively known as dithiocarbamates (Figure 2), are ultimately excreted in the urine (4).
Bioavailability
The composition and content of glucosinolates in cruciferous vegetables are relatively stable but depend on the genus and species and can vary with plant growing and post-harvest storage conditions and culinary processing (6, 7). Since most cruciferous vegetables are cooked prior to eating, bacterial myrosinase in the gut, rather than plant myrosinase, is responsible for the initial step in glucosinolate degradation (Figure 2). In a feeding study involving 45 healthy subjects, the mean conversion rate of glucosinolates (of which 85% was glucoraphanin) to dithiocarbamates over a 24-hour period was estimated to be around 12% with wide variations among participants (range, 1.1 to 40.7%) (6). In contrast, 70%-75% of ingested isothiocyanates were found to be metabolized to dithiocarbamates. Therefore, following the ingestion of cooked cruciferous vegetables, the conversion of glucosinolates into isothiocyanates by gut bacteria appears to be a limiting step in the generation of dithiocarbamates (6). However, differences in individuals’ capacity to metabolize glucosinolates have not been linked to differences in gut microbiota composition (8).
Biological Activities
Antioxidant activity
Many isothiocyanates, particularly sulforaphane, have been shown to induce the expression of antioxidant enzymes via the activation of the nuclear factor E2-related factor 2 (Nrf2)-dependent pathway (9, 10). Briefly, Nrf2 is a transcription factor that is bound to the protein Kelch-like ECH-associated protein 1 (Keap1) in the cytosol (Figure 3). Keap1 responds to oxidative stress signals or chemical inducers by freeing Nrf2. Isothiocyanates can react with sulfhydryl residues of Keap1, causing the release of Nrf2. Nrf2 can then translocate to the nucleus and bind to the antioxidant response element (ARE) located in the promoters of genes coding for antioxidant/detoxifying enzymes and scavengers. Nrf2/ARE-dependent genes code for several mediators of the antioxidant response, including glutathione S-transferases (GSTs), thioredoxin, NAD(P)H quinone oxidoreductase 1 (NQO-1), and heme oxygenase 1 (HO-1) (11).
In numerous animal models, sulforaphane (often administered ip, iv, or sc, rather than po) was shown to exert protective effects on many tissues and organs by activating the Nrf2/ARE-dependent pathway (12). For example, sulforaphane reduced contrast agent-induced kidney damage in rats by increasing Nrf2 nuclear translocation and upregulating the expression of HO-1 and NQO-1 (13). Upregulation of the Nrf2 pathway by sulforaphane also attenuated oxidative damage-induced vascular endothelial cell injury in a mouse model of type 2 diabetes mellitus (14). In a rat model of hepatic ischemia reperfusion injury — whereby cellular damage is caused by the restoration of oxygen delivery to a hypoxic liver — pre-treatment with sulforaphane limited the reduction in glutathione (GSH) and the antioxidant enzymes, superoxide dismutase (SOD) and GSH peroxidase (GPx). Sulforaphane also upregulated the expression of Nrf2, NQO-1, and HO-1, and decreased ischemic death and apoptosis of liver cells (15).
Human studies are limited. In a placebo-controlled study, oral sulforaphane (in the form of broccoli sprout homogenate) increased the expression of NQO-1 and HO-1 in the upper airway within two hours of ingestion (16). Yet, in a recent trial in patients with chronic obstructive pulmonary disease (COPD), oral administration of sulforaphane for four weeks failed to induce the expression of Nrf2, NQO-1, and HO-1 in alveolar macrophages, bronchial epithelial cells, or peripheral blood mononuclear cells (17).
Anti-inflammatory activity
The therapeutic potential of sulforaphane has also been linked to its capacity to target pro-inflammatory pathways. Sulforaphane was found to attenuate pancreatic injury in a mouse model of acute pancreatitis by stimulating Nrf2-induced antioxidant enzymes (18). Concomitantly, sulforaphane significantly reduced the nuclear translocation of the pro-inflammatory transcription factor nuclear factor (NF)-κB in pancreatic acinar cells, downregulating the expression of NF-κB target genes that code for pro-inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1β), and IL-6 (Figure 3) (18). Through inhibiting the NF-κB pathway, sulforaphane also targets other mediators of the inflammatory response, including the enzymes cyclooxygenase-2 (COX-2), prostaglandin E (PGE) synthase, and inducible nitric oxide synthase (iNOS). Sulforaphane exhibited anti-inflammatory effects in the lungs of mice with lipopolysaccharide (LPS)-induced acute respiratory distress syndrome (ARDS) by downregulating the expression of NF-κB, IL-6, TNF-α and COX-2, as well as decreasing production of nitric oxide (NO) and PGE2 (19). Other isothiocyanates have been shown to prevent the degradation of the NF-κB inhibitor, IκB, the nuclear translocation of NF-κB, and/or the transcriptional activity of NF-κB in vitro or in cultured cells (Figure 3) (20), which all can lead to a decrease in inflammatory responses.
The modulation of Nrf2 and NF-κB signaling pathways by isothiocyanates is especially relevant to the prevention of cancer because both oxidative stress and inflammation are significant contributors in the development and progression of cancer.
Anticancer activity
Biotransformation enzymes play important roles in the metabolism and elimination of a variety of chemicals, including drugs, toxins, and carcinogens. In general, phase I metabolizing enzymes catalyze reactions that increase the reactivity of hydrophobic (fat-soluble) compounds, preparing them for reactions catalyzed by phase II biotransformation enzymes. Reactions catalyzed by phase II enzymes generally increase water solubility and promote the elimination of the compound from the body (21).
Inhibition of phase I biotransformation enzymes
Isothiocyanates have been found to modulate the activity of phase I biotransformation enzymes, especially those of the cytochrome P450 (CYP) family. Using a primary rat hepatocyte-based model, both aliphatic (e.g., sulforaphane, AITC) and aromatic (e.g., BITC, PEITC) isothiocyanates (at 20-40 μM) have been found to downregulate CYP3A2 mRNA expression, as well as the activity of benzyloxyquinoline debenzylase, a marker of CYP3As (22). Aromatic isothiocyanates were also able to upregulate CYP1A1 and CYP1A2 mRNA expression and the activity of ethoxyresorufin-O-deethylase (EROD), a marker of CYP1A1/2 activities (22). In this model, sulforaphane inhibited EROD activity, yet failed to affect CYP1A1/2 mRNA expression (22). Using human liver microsomes, it was also recently reported that sulforaphane metabolites (0-200 μM) had little-to-no effect on the activities of CYP1A2, CYP2B6, CYP2D6, and CYP3A4 (23). The ability of PEITC to alter the expression and activity of CYP enzymes has been generally associated with a protective effect against (pro)carcinogen-induced tumor development in animal experiments (reviewed in 24). Increasing the activity of biotransformation enzymes may be beneficial if the elimination of potential carcinogens or toxins is enhanced. Yet, some procarcinogens require phase I enzymes in order to become active carcinogens capable of binding DNA and forming cancer-causing DNA adducts. Inhibition of specific CYP enzymes involved in carcinogen activation has been found to prevent the development of cancer in animal models (3).
Induction of phase II detoxifying enzymes
Many isothiocyanates are potent inducers of phase II detoxifying enzymes, including GSTs, UDP-glucuronosyl transferases (UGTs), NQO1, and glutamate cysteine ligase (GCL), that protect cells from DNA damage by carcinogens and reactive oxygen species (ROS) (25). The genes for these and other phase II enzymes contain AREs and are therefore under the control of Nrf2 (see Antioxidant activity). Limited data from clinical trials suggest that glucosinolate-rich foods can increase phase II enzyme activity in humans. When smokers consumed 170 g/day (6 oz/day) of watercress, urinary excretion of glucuronidated nicotine metabolites increased significantly, suggesting UGT activity increased (26). Brussels sprouts are rich in a number of glucosinolates, including precursors of AITC and sulforaphane. Consumption of 300 g/day (11 oz/day) of Brussels sprouts for one week significantly increased plasma and intestinal GST concentrations in nonsmoking men (27, 28).
Induction of cell cycle arrest and apoptosis
After a cell divides, it passes through a sequence of stages — collectively known as the cell cycle — before dividing again. Following DNA damage, the cell cycle can be transiently arrested to allow for DNA repair or activation of pathways leading to programmed cell death (apoptosis) when the damage cannot be repaired (29). Defective cell cycle regulation and pro-survival mechanisms may result in the propagation of mutations that contribute to the development of cancer. Isothiocyanates have been found to modulate the expression of the cell cycle regulators, cyclins and cyclin-dependent kinases (CDK), as well as trigger apoptosis in a number of cancer cell lines (20). In a mouse model of colorectal cancer, oral administration of PEITC reduced both the number and size of polyps; these changes were associated with activation of the CDK inhibitor, p21, inhibition of various cyclins (A, D1, and E), and induction of apoptosis (30). In a transgenic prostate adenocarcinoma mouse model, BITC limited the progress of prostatic intraepithelial neoplasia (PIN) to a well-differentiated carcinoma (31). This was related to a decreased expression of Ki67 (a marker of cell proliferation) and a downregulation of cyclin A, cyclin D1, and CDK2, which regulate cell cycle progression (31).
Inhibition of cell migration and invasion
The epithelial-to-mesenchymal transition (EMT) describes a process of epithelial cell transformation whereby cells lose their polarity and adhesion properties while gaining migratory and invasive properties through the expression of mesenchymal genes. Inhibition of the EMT by sulforaphane in thyroid cancer cells has been associated with upregulation of an epithelial marker, E-cadherin, and downregulation of a transcription factor (SNAI2), a filament protein (vimentin), and various enzymes (matrix metalloprotein [MMP]-2 and MMP-9) known to contribute to EMT and promote migration (32). In a xenograft mouse model of breast cancer, BITC inhibited high fat diet-driven promotion of breast tumor growth, as well as lung and liver metastasis (33). This study suggested that BITC might prevent the infiltration of macrophages in the tumor environment (33). In another model of breast tumor metastasis, PEITC inhibited the migration of tumor cells to the brain after injection into the heart of mice, limiting the growth of metastatic brain tumors (34).
Inhibition of angiogenesis
To fuel their rapid growth, invasive tumors must also develop new capillaries from preexisting blood vessels by a process known as angiogenesis. Isothiocyanates have been shown to prevent the formation of capillary-like structures from human umbilical endothelial cells (reviewed in 35). Isothiocyanates likely inhibit the expression and function of hypoxia inducible factors (HIFs) that control angiogenesis, as reported in endothelial cells and malignant cell lines (35).
Epigenetic regulation of gene expression
In the nucleus of a cell, DNA is coiled around proteins called histones, thereby forming the chromatin. The N-terminal tails of histones are targets for multiple modifications, including phosphorylation, methylation, acetylation, ubiquitination, poly ADP ribosylation, and sumoylation. Histone modification patterns have differential effects on chromatin structure, and, in synergy with DNA methylation, are implicated in the regulating expression of the genome (36). Within gene regulatory regions, the acetylation of lysine residues of histone tails has been correlated with activation of transcription. Conversely, the deacetylation of histones by histone deacetylases (HDAC) restricts access of transcription factors to the DNA and suppresses transcription. Because abnormal epigenetic marks disrupt the expression of specific tumor suppressor genes in cancer cells, compounds that re-induce their transcription, like those inhibiting HDACs, can potentially promote differentiation and apoptosis in transformed (precancerous) cells (37).
Isothiocyanates have been found to inhibit HDAC expression and/or activity in cultured cancer cells (38-43). Moreover, in vivo evidence for HDAC inhibition by sulforaphane came from a mouse model using prostate cancer xenografts (44). In humans, HDAC activity was reduced in blood cells following ingestion of 68 g (one cup) of sulforaphane-rich broccoli sprouts (44). Isothiocyanates may also affect microRNA-mediated gene silencing. In bladder cancer cells, E-cadherin induction by sulforaphane was partly due to the upregulation of miR-200c expression resulting in the miR-200c-dependent suppression of ZEB-1, a transcriptional repressor of E-cadherin (45). PEITC inhibited androgen receptor (AR) transcriptional activity in prostate cancer cells by repressing miR-141 expression and miR-141-mediated downregulation of small heterodimer partner (shp), a repressor of AR (46).
Antibacterial activity
Bacterial infection with Helicobacter pylori is associated with a marked increase in the risk of peptic ulcer disease and gastric cancer (47). In the test tube and in tissue culture, purified sulforaphane inhibited the growth and killed multiple strains of H. pylori, including antibiotic resistant strains (48). In an animal model of H. pylori infection, sulforaphane administration for five days eradicated H. pylori from 8 out of 11 xenografts of human gastric tissue implanted in immune-compromised mice (49). In another H. pylori-infected mouse model, a functional Nrf2 pathway was found to be required for the reduction of gastric inflammation and infection in mice fed broccoli sprouts (50). In a small clinical trial, consumption of up to 56 g/day (2 oz/day) of glucoraphanin-rich broccoli sprouts for one week was associated with H. pylori eradication in only three out of nine gastritis patients (51). In another trial, daily consumption of 70 g/day (~2-3 servings/day) of glucoraphanin-rich broccoli sprouts for two months significantly reduced markers of inflammation and infection in H. pylori–infected volunteers compared to those who consumed alfalfa sprouts (50). However, the extent to which glucoraphanin was converted to sulforaphane in broccoli sprout-fed participants was not measured.
Disease Prevention
Cancer
Isothiocyanates are thought to play a prominent role in the potential anticancer and cardiovascular benefits associated with cruciferous vegetable consumption (52, 53). Genetic variations in the sequence of genes coding for GSTs may affect the activity of GSTs. Such variations have been identified in humans. Specifically, null variants of the GSTM1 and GSTT1 alleles contain large deletions, and individuals who inherit two copies of the GSTM1-null or GSTT1-null alleles cannot produce the corresponding GST enzymes (54). It has been proposed that a reduced GST activity in these individuals would slow the rate of excretion of isothiocyanates, thereby increasing tissue exposure to isothiocyanates after cruciferous vegetable consumption (55). In addition, GSTs are involved in "detoxifying" potentially harmful substances like carcinogens, suggesting that individuals with reduced GST activity might also be more susceptible to cancer (56-58). Further, induction of the expression and activity of GSTs and other phase II detoxification/antioxidant enzymes by isothiocyanates is an important defense mechanism against oxidative stress and damage associated with the development of diseases like cancer and cardiovascular disease (11). The ability of glucoraphanin-derived sulforaphane to reduce oxidative stress in different settings is linked to activation of the nuclear factor E2-related factor 2 (Nrf2)-dependent pathway (see Biological Activities). Yet, whether potential protection conferred by isothiocyanates via the Nrf2-dependent pathway is diminished in individuals carrying GST null variants is currently unknown. Some, but not all, observational studies have suggested that GST genotypes could influence the associations between cruciferous vegetable consumption and risk of disease (59).
Naturally occurring isothiocyanates and their metabolites have been found to inhibit the development of chemically-induced cancers of the lung, liver, esophagus, stomach, small intestine, colon, and breast in a variety of animal models (20). Although observational studies provide some evidence that higher intakes of cruciferous vegetables are associated with decreased cancer risk in humans (59), it is difficult to determine whether such protective effects are related to isothiocyanates or other factors associated with cruciferous vegetable consumption (see the article on Cruciferous Vegetables). Clinical evidence of a protective effect of isothiocyanates in humans is scarce. For example, in a recent randomized, cross-over intervention, administration of PEITC (40 mg/day for five days) caused a modest, yet significant, 7.7% reduction in the metabolic activation of the tobacco-specific lung carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, in cigarette smokers (60). Another randomized controlled trial in men with biochemically relapsing cancer after radical prostatectomy suggested that prostate-specific antigen (PSA) values tended to increase less in those given daily oral sulforaphane (4.4 or 26.6 mg/day) for six months compared to those receiving the placebo (61). In a recent double-blind, randomized, placebo-controlled trial in women with abnormal mammograms, two-to-eight week consumption of about 250 mg/day of broccoli seed extract (~220 mg of glucoraphanin/day) before surgery failed to affect the expression of markers of proliferation and gene expression, including ki-67, p21, HDACs, and acetylated histones, in breast tissues collected after surgery (62).
Sources
Food sources
Cruciferous vegetables
Cruciferous vegetables, such as bok choi, broccoli, Brussels sprouts, cabbage, cauliflower, horseradish, kale, kohlrabi, mustard, radish, rutabaga, turnip, and watercress, are rich sources of glucosinolate precursors of isothiocyanates (63). Unlike some other phytochemicals, glucosinolates are present in relatively high concentrations in commonly consumed portions of cruciferous vegetables. For example one-half cup of raw broccoli might provide more than 25 mg of total glucosinolates. The glucosinolate content of selected cruciferous vegetables is presented in Table 1 (64). Note that while the composition and content of glucosinolates in cruciferous vegetables are relatively stable, they depend on the genus and species and can vary greatly with plant growing and post-harvest storage conditions, as well as culinary processing.
Table 2 lists vegetables that are relatively good sources of some of the isothiocyanates that are currently being studied for their potential anticancer properties (65).
Amounts of isothiocyanates formed from glucosinolates in foods are variable and depend partly on food processing and preparation (see the article on Cruciferous Vegetables). In a recent study that examined total isothiocyanate content in 73 samples from nine types of raw cruciferous vegetables commonly consumed in the US (namely broccoli, cabbage, cauliflower, Brussels sprout, kale, collard green, mustard green, and turnip greens), an average yield of 16.2 µmol/100 g wet weight was reported, with a 41-fold difference of isothiocyanate yield across the vegetables. The lowest mean level of isothiocyanate yield was found with raw cauliflower (1.5 µmol/100 g), while raw mustard greens had the highest yield (61.3 µmol/100g) (66).
Broccoli sprouts
The amount of glucoraphanin, the precursor of sulforaphane, in broccoli seeds remains more or less constant as those seeds germinate and grow into mature plants. Thus, three-day old broccoli sprouts are concentrated sources of glucoraphanin, which contain 10 to 100 times more glucoraphanin by weight than mature broccoli plants (67). Broccoli sprouts that are certified to contain at least 73 mg of glucoraphanin (also called sulforaphane glucosinolate) per 1-oz serving are available in some health food and grocery stores.
Supplements
Dietary supplements containing extracts of broccoli sprouts, broccoli, and other cruciferous vegetables are available without a prescription. Some products are standardized to contain a minimum amount of glucosinolates and/or sulforaphane. However, the bioavailability of isothiocyanates was found to be much lower with the consumption of broccoli supplements devoid of myrosinase than with the consumption of fresh broccoli sprouts. Peak concentrations of sulforaphane metabolites were found to be eight- and five-times greater in plasma and urine, respectively, following fresh broccoli versus supplement consumption (68). Interestingly, total HDAC activity in peripheral blood mononuclear cells (PBMC) of broccoli sprout consumers was reported to be significantly lower than in PBMC of subjects who consumed the supplement (see Biological Activities) (69).
Safety
Adverse effects
No serious adverse effects of isothiocyanates in humans have been reported. The majority of animal studies have found that isothiocyanates inhibited the development of cancer when given prior to the chemical carcinogen (pre-initiation). However, very high intakes of PEITC or BITC (25 to 250 times higher than average human dietary isothiocyanate intakes) have been found to promote bladder cancer in rats when given after cancer initiation by a chemical carcinogen (70). The relevance of these findings to human urinary bladder cancer is not clear, since at least one prospective cohort study found cruciferous vegetable consumption to be inversely associated with the risk of bladder cancer in men (71). Other potential toxic effects reported in rodents have not been corroborated by observations in humans (20).
Pregnancy and lactation
Although high dietary intakes of glucosinolates from cruciferous vegetables are not known to have adverse effects during pregnancy or lactation, there is no information on the safety of purified isothiocyanates or supplements containing high doses of glucosinolates and/or isothiocyanates during pregnancy or lactation in humans.
Drug interactions
Isothiocyanates are not known to interact with any drugs or medications. However, the potential for isothiocyanates to inhibit various isoforms of the cytochrome P450 (CYP) family of enzymes raises the potential for interactions with drugs that are CYP substrates (see Biological Activities). Isothiocyanates may sensitize cancer cells to anticancer drugs and/or increase drug cytotoxicity, as shown in in vitro and animal models. Yet, these potential benefits of isothiocyanates in cancer therapy have not been explored in clinical trials (72).
Authors and Reviewers
Originally written in 2005 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2008 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in March 2017 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in April 2017 by:
Emily Ho, Ph.D.
Principal Investigator, Linus Pauling Institute
Professor, College of Public Health and Human Sciences
Endowed Director, Moore Family Center for Whole Grain Foods,
Nutrition and Preventive Health
Oregon State University
Copyright 2005-2024 Linus Pauling Institute
References
1. Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5-51. (PubMed)
2. Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555(1-2):173-190. (PubMed)
3. Hecht SS. Chemoprevention by Isothiocyanates. In: Kelloff GJ, Hawk ET, Sigman CC, eds. Promising Cancer Chemopreventive Agents, Volume 1: Cancer Chemopreventive Agents Totowa, NJ: Humana Press; 2004:21-35.
4. Barba FJ, Nikmaram N, Roohinejad S, Khelfa A, Zhu Z, Koubaa M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front Nutr. 2016;3:24. (PubMed)
5. Luang-In V, Albaser AA, Nueno-Palop C, Bennett MH, Narbad A, Rossiter JT. Glucosinolate and Desulfo-glucosinolate Metabolism by a Selection of Human Gut Bacteria. Curr Microbiol. 2016;73(3):442-451. (PubMed)
6. Fahey JW, Wehage SL, Holtzclaw WD, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila). 2012;5(4):603-611. (PubMed)
7. Verkerk R, Schreiner M, Krumbein A, et al. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. 2009;53 Suppl 2:S219. (PubMed)
8. Li F, Hullar MA, Beresford SA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr. 2011;106(3):408-416. (PubMed)
9. Hu R, Xu C, Shen G, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79(20):1944-1955. (PubMed)
10. Wagner AE, Boesch-Saadatmandi C, Dose J, Schultheiss G, Rimbach G. Anti-inflammatory potential of allyl-isothiocyanate--role of Nrf2, NF-(kappa) B and microRNA-155. J Cell Mol Med. 2012;16(4):836-843. (PubMed)
11. Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85(6):705-717. (PubMed)
12. Guerrero-Beltran CE, Calderon-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64(5):503-508. (PubMed)
13. Zhao Z, Liao G, Zhou Q, Lv D, Holthfer H, Zou H. Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid Med Cell Longev. 2016;2016:9825623. (PubMed)
14. Wang Y, Zhang Z, Sun W, et al. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev. 2014;2014:123963. (PubMed)
15. Chi X, Zhang R, Shen N, et al. Sulforaphane reduces apoptosis and oncosis along with protecting liver injury-induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol Int. 2015;9(2):321-329. (PubMed)
16. Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130(3):244-251. (PubMed)
17. Wise RA, Holbrook JT, Criner G, et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PLoS One. 2016;11(11):e0163716. (PubMed)
18. Dong Z, Shang H, Chen YQ, Pan LL, Bhatia M, Sun J. Sulforaphane protects pancreatic acinar cell injury by modulating Nrf2-mediated oxidative stress and NLRP3 inflammatory pathway. Oxid Med Cell Longev. 2016;2016:7864150. (PubMed)
19. Qi T, Xu F, Yan X, Li S, Li H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int J Mol Med. 2016;37(1):182-188. (PubMed)
20. Kumar G, Tuli HS, Mittal S, Shandilya JK, Tiwari A, Sandhu SS. Isothiocyanates: a class of bioactive metabolites with chemopreventive potential. Tumour Biol. 2015;36(6):4005-4016. (PubMed)
21. Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132(10):2991-2994. (PubMed)
22. La Marca M, Beffy P, Della Croce C, et al. Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem Toxicol. 2012;50(8):2822-2830. (PubMed)
23. Vanduchova A, Tomankova V, Anzenbacher P, Anzenbacherova E. Influence of Sulforaphane Metabolites on Activities of Human Drug-Metabolizing Cytochrome P450 and Determination of Sulforaphane in Human Liver Cells. J Med Food. 2016;19(12):1141-1146. (PubMed)
24. Ioannides C, Konsue N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab Rev. 2015;47(3):356-373. (PubMed)
25. Kensler TW, Talalay P. Inducers of enzymes that protect against carcinogens and oxidants: drug- and food-based approaches with dithiolethiones and sulforaphane. In: Kelloff GJ, Hawk ET, Sigman CC, eds. Promising Cancer Chemopreventive Agents, Volume 1: Cancer Chemopreventive Agents Totowa, NJ: Humana Press; 2004:3-20.
26. Hecht SS, Carmella SG, Murphy SE. Effects of watercress consumption on urinary metabolites of nicotine in smokers. Cancer Epidemiol Biomarkers Prev. 1999;8(10):907-913. (PubMed)
27. Nijhoff WA, Grubben MJ, Nagengast FM, et al. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis. 1995;16(9):2125-2128. (PubMed)
28. Nijhoff WA, Mulder TP, Verhagen H, van Poppel G, Peters WH. Effects of consumption of brussels sprouts on plasma and urinary glutathione S-transferase class-alpha and -pi in humans. Carcinogenesis. 1995;16(4):955-957. (PubMed)
29. Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. 2003;24(3):139-145. (PubMed)
30. Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN. Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC). Mol Carcinog. 2008;47(5):321-325. (PubMed)
31. Cho HJ, Lim do Y, Kwon GT, et al. Benzyl isothiocyanate inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate (TRAMP) model, which is associated with the induction of cell cycle G1 arrest. Int J Mol Sci. 2016;17(2):264. (PubMed)
32. Wang L, Tian Z, Yang Q, et al. Sulforaphane inhibits thyroid cancer cell growth and invasiveness through the reactive oxygen species-dependent pathway. Oncotarget. 2015;6(28):25917-25931. (PubMed)
33. Kim M, Cho HJ, Kwon GT, et al. Benzyl isothiocyanate suppresses high-fat diet-stimulated mammary tumor progression via the alteration of tumor microenvironments in obesity-resistant BALB/c mice. Mol Carcinog. 2015;54(1):72-82. (PubMed)
34. Gupta P, Adkins C, Lockman P, Srivastava SK. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS One. 2013;8(6):e67278. (PubMed)
35. Cavell BE, Syed Alwi SS, Donlevy A, Packham G. Anti-angiogenic effects of dietary isothiocyanates: mechanisms of action and implications for human health. Biochem Pharmacol. 2011;81(3):327-336. (PubMed)
36. Delage B, Dashwood RH. Targeting the epigenome with dietary agents. Dietary Modulation of Cell Signaling Pathways: CRC Press; 2008.
37. Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137-168. (PubMed)
38. Abbaoui B, Telu KH, Lucas CR, et al. The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer. J Proteomics. 2017;156:94-103. (PubMed)
39. Batra S, Sahu RP, Kandala PK, Srivastava SK. Benzyl isothiocyanate-mediated inhibition of histone deacetylase leads to NF-kappaB turnoff in human pancreatic carcinoma cells. Mol Cancer Ther. 2010;9(6):1596-1608. (PubMed)
40. Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55(7):999-1009. (PubMed)
41. Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6(3):1013-1021. (PubMed)
42. Rajendran P, Delage B, Dashwood WM, et al. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011;10:68. (PubMed)
43. Rajendran P, Kidane AI, Yu TW, et al. HDAC turnover, CtIP acetylation and dysregulated DNA damage signaling in colon cancer cells treated with sulforaphane and related dietary isothiocyanates. Epigenetics. 2013;8(6):612-623. (PubMed)
44. Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood). 2007;232(2):227-234. (PubMed)
45. Shan Y, Zhang L, Bao Y, et al. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J Nutr Biochem. 2013;24(6):1062-1069. (PubMed)
46. Xiao J, Gong AY, Eischeid AN, et al. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate. 2012;72(14):1514-1522. (PubMed)
47. US National Cancer Institute. Helicobacter pylori and cancer. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/h-pylori-fact-sheet. Accessed 2/28/17.
48. Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99(11):7610-7615. (PubMed)
49. Haristoy X, Angioi-Duprez K, Duprez A, Lozniewski A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob Agents Chemother. 2003;47(12):3982-3984. (PubMed)
50. Yanaka A, Fahey JW, Fukumoto A, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila). 2009;2(4):353-360. (PubMed)
51. Galan MV, Kishan AA, Silverman AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci. 2004;49(7-8):1088-1090. (PubMed)
52. Bai Y, Wang X, Zhao S, Ma C, Cui J, Zheng Y. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxid Med Cell Longev. 2015;2015:407580. (PubMed)
53. Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224-236. (PubMed)
54. Coles BF, Kadlubar FF. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors. 2003;17(1-4):115-130. (PubMed)
55. Seow A, Shi CY, Chung FL, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7(9):775-781. (PubMed)
56. Economopoulos KP, Choussein S, Vlahos NF, Sergentanis TN. GSTM1 polymorphism, GSTT1 polymorphism, and cervical cancer risk: a meta-analysis. Int J Gynecol Cancer. 2010;20(9):1576-1580. (PubMed)
57. Egner PA, Chen JG, Zarth AT, et al. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila). 2014;7(8):813-823. (PubMed)
58. Yuan JM, Murphy SE, Stepanov I, et al. 2-Phenethyl Isothiocyanate, Glutathione S-transferase M1 and T1 Polymorphisms, and Detoxification of Volatile Organic Carcinogens and Toxicants in Tobacco Smoke. Cancer Prev Res (Phila). 2016;9(7):598-606. (PubMed)
59. Traka MH. Chapter 9: Health benefits of glucosinolates. Advances in Botanical Research. 2016;80:247-279.
60. Yuan JM, Stepanov I, Murphy SE, et al. Clinical trial of 2-phenethyl isothiocyanate as an inhibitor of metabolic activation of a tobacco-specific lung carcinogen in cigarette smokers. Cancer Prev Res (Phila). 2016;9(5):396-405. (PubMed)
61. Cipolla BG, Mandron E, Lefort JM, et al. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res (Phila). 2015;8(8):712-719. (PubMed)
62. Atwell LL, Zhang Z, Mori M, et al. Sulforaphane bioavailability and chemopreventive activity in women scheduled for breast biopsy. Cancer Prev Res (Phila). 2015;8(12):1184-1191. (PubMed)
63. International Agency for Research on Cancer. Cruciferous vegetables, isothiocyanates and indoles. Cruciferous vegetables. France: IARC; 2004:1-12.
64. McNaughton SA, Marks GC. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr. 2003;90(3):687-697. (PubMed)
65. Ishida M, Hara M, Fukino N, Kakizaki T, Morimitsu Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed Sci. 2014;64(1):48-59. (PubMed)
66. Tang L, Paonessa JD, Zhang Y, Ambrosone CB, McCann SE. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J Funct Foods. 2013;5(4):1996-2001. (PubMed)
67. Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94(19):10367-10372. (PubMed)
68. Clarke JD, Hsu A, Riedl K, et al. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res. 2011;64(5):456-463. (PubMed)
69. Clarke JD, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem. 2011;59(20):10955-10963. (PubMed)
70. Okazaki K, Umemura T, Imazawa T, Nishikawa A, Masegi T, Hirose M. Enhancement of urinary bladder carcinogenesis by combined treatment with benzyl isothiocyanate and N-butyl-N-(4-hydroxybutyl)nitrosamine in rats after initiation. Cancer Sci. 2003;94(11):948-952. (PubMed)
71. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91(7):605-613. (PubMed)
72. Minarini A, Milelli A, Fimognari C, Simoni E, Turrini E, Tumiatti V. Exploring the effects of isothiocyanates on chemotherapeutic drugs. Expert Opin Drug Metab Toxicol. 2014;10(1):25-38. (PubMed)

![Figure 3. Isothiocyanates Target Nrf2 and NF-kappaB Pathways. Isothiocyanates inhibit NF-kappaB-mediated inflammation and increase the expression of phase II detoxifying/antioxidant enzymes via the Nrf2 signaling pathway. [A] Isothiocyanates induce the nuclear translocation of Nrf2 and increase the expression of Nrf2 target genes coding for phase II enzymes and antioxidant enzymes. [B] Isothiocyanates may prevent (1) the phosphorylation of NF-kappaB inhibitor, IkappaB; (2) th nuclear translocation of NF-kappaB; and (3) the transcriptional activity of NF-kappa B.](/sites/lpi.oregonstate.edu/files/isothiocyanates-figure-3-800px.png)