Contents
Flavonoids are a large family of over 5,000 hydroxylated polyphenolic compounds that carry out important functions in plants, including attracting pollinating insects; combating environmental stresses, such as microbial infection; and regulating cell growth (1). Their bioavailability and biological activities in humans appear to be strongly influenced by their chemical nature. Since the 1990s, there has been a growing interest in dietary flavonoids due to their likely contribution to the health benefits of fruit- and vegetable-rich diets. This article reviews some of the scientific evidence regarding the role of dietary flavonoids in health promotion and disease prevention in humans; it is not meant to be a comprehensive review on every health topic studied.
Flavonoids are classified into 12 major subclasses based on chemical structures, six of which, namely anthocyanidins, flavan-3-ols, flavonols, flavones, flavanones, and isoflavones (Table 1 and Figures 1-9) are of dietary significance. Glycosylated flavonols (bound to at least one sugar molecule) are the most widely distributed flavonoids in the diet (2, 3).
| Flavonoid Subclass | Dietary Flavonoids (aglycones) | Some Common Food Sources (see also Sources) |
|---|---|---|
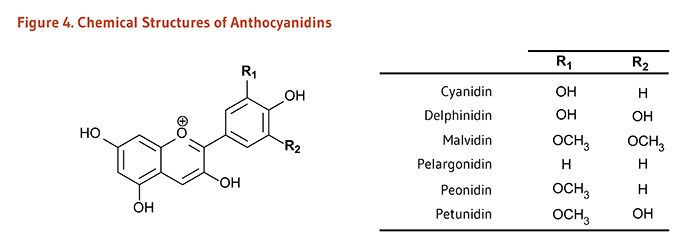
| Anthocyanidins* | Cyanidin, Delphinidin, Malvidin, Pelargonidin, Peonidin, Petunidin | Red, blue, and purple berries; red and purple grapes; red wine |
|
|
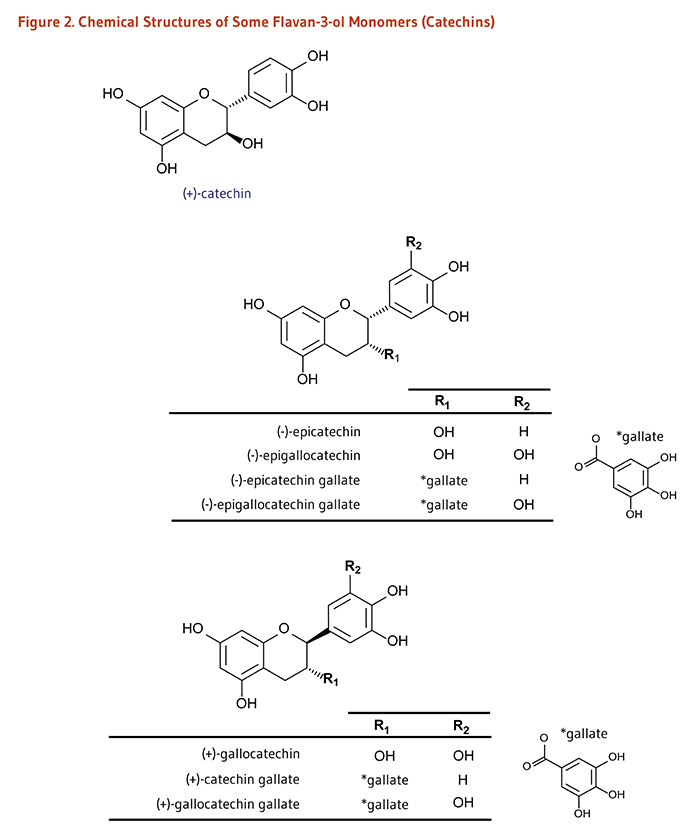
Monomers (Catechins): (+)-Catechin, (-)-Epicatechin, (-)-Epigallocatechin, (+)-Gallocatechin; and their gallate derivatives |
Teas (particularly white, green, and oolong), cocoa-based products, grapes, berries, apples |
|
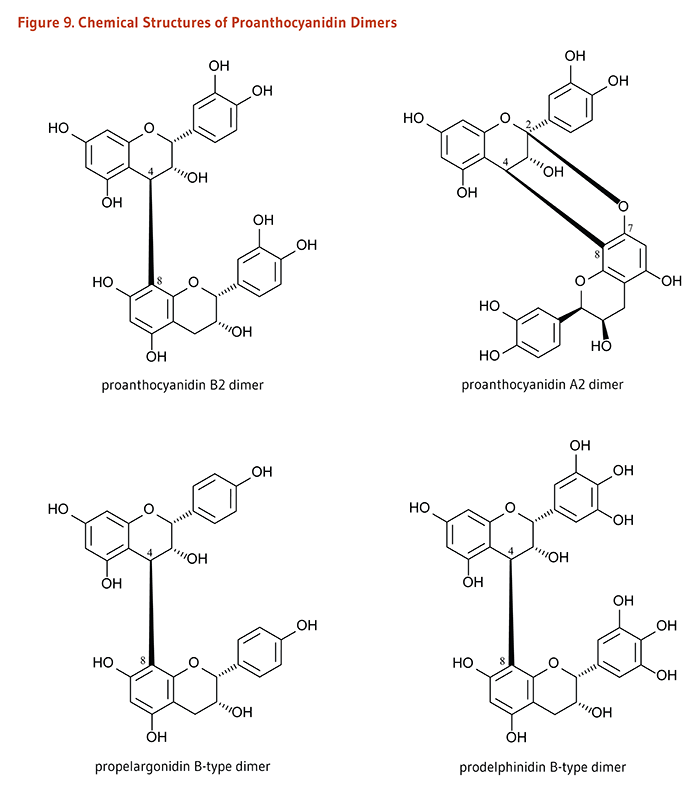
Dimers and Polymers: Proanthocyanidins# |
Apples, berries, cocoa-based products, red grapes, red wine |
|
| Theaflavins, Thearubigins |
Black tea |
|
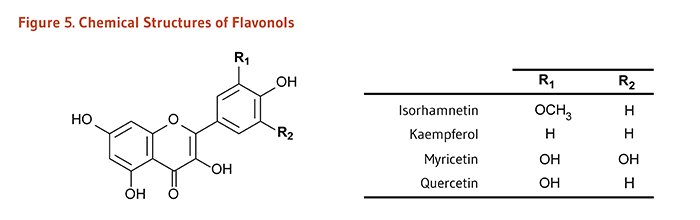
| Flavonols | Isorhamnetin, Kaempferol, Myricetin, Quercetin |
Onions, scallions, kale, broccoli, apples, berries, teas |
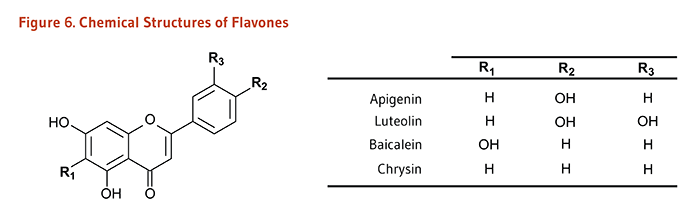
| Flavones | Apigenin, Luteolin, Baicalein, Chrysin |
Parsley, thyme, celery, hot peppers |
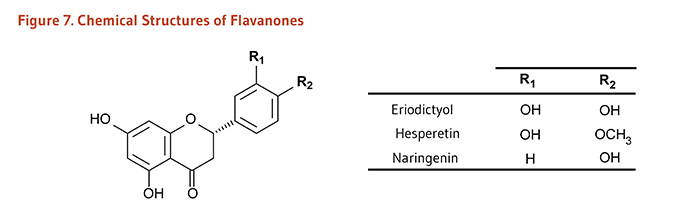
| Flavanones | Eriodictyol, Hesperetin, Naringenin |
Citrus fruit and juices, e.g., oranges, grapefruits, lemons |
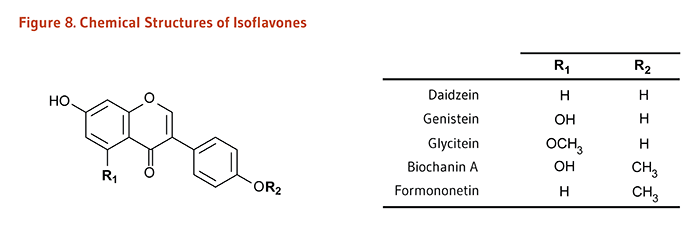
| Isoflavones |
Daidzein, Genistein, Glycitein, Biochanin A, Formononetin |
Soybeans, soy foods, legumes |
|
*Anthocyanidins with one or more sugar moieties (anthocyanidin glycosides) are called anthocyanins. #Proanthocyanidin oligomers formed from (+)-catechin and (-)-epicatechin subunits are called procyanidins. |
||
For more detailed information on the health effects of isoflavones, a subclass of flavonoids with estrogenic activity, see the article on Soy Isoflavones.
For more information on the health benefits of foods that are rich in flavonoids, see the articles on Fruit and Vegetables, Legumes, and Tea.


The amount of flavonoids present in ingested food has little importance unless dietary flavonoids are absorbed and become available to target tissues within the body. During and after intestinal absorption, flavonoids are rapidly and extensively metabolized in intestinal and liver cells such that they are likely to appear as metabolites (e.g., phase II metabolites) in the bloodstream and urine (4). Additionally, the biological activities of flavonoid metabolites are likely to be different from those of their parent compounds (5). Some of the factors influencing the metabolic fate and bioavailability of dietary flavonoids are mentioned below.
Most flavonoids occur in edible plants and foods as β-glycosides, i.e., bound to one or more sugar molecules (6). Exceptions include flavan-3-ols (catechins and proanthocyanidins) and fermented soy-based products that are exposed to microbial β-glucosidases, which catalyze the release of sugar molecules from glycosylated isoflavones (7). Even after food processing and cooking, most flavonoid glycosides reach the small intestine intact. Only flavonoid aglycones (not bound to a sugar molecule) and a few flavonoid glucosides (bound to glucose) are easily absorbed in the small intestine (8). Glycosylated flavonoids might be able to penetrate the mucus layer of the intestine and be deglycosylated on the cell surface before absorption. Those that cannot be deglycosylated in the small intestine may be hydrolyzed by bacterial enzymes in the colon (7). Nevertheless, colonic bacteria might remove sugar moieties and rapidly degrade aglycone flavonoids, thus limiting their absorption in the colon (9).
In contrast to monomeric flavan-3-ols (catechins), the polymeric nature of proanthocyanidins likely prevents their intestinal absorption. Flavan-3-ol monomers and procyanidins are transformed by the intestinal microbiota to 5-(hydroxyphenyl)-γ-valerolactones which appear in the circulatory system and are excreted in urine as sulfate and glucuronide metabolites (Figure 10). Valerolactones may be further degraded by the colonic microbiota to smaller phenolic acids and aromatic compounds. The colonic microbiota also metabolize the gallate esters of flavonoids, generating gallate, which is further catabolized to pyrogallol. Microbe-derived flavonoid metabolites are readily absorbed into the circulatory system and excreted in both free forms and as phase II metabolites in urine (9).

The presence of macronutrients in food influences the bioavailability of co-ingested flavonoids (reviewed in 8, 10, 11). The binding affinity and potential (non-) covalent interactions of flavonoids with food proteins, carbohydrates, and fats are directly associated with the physicochemical properties of flavonoids (reviewed in 8). Proteins in milk might reduce the absorption of polyphenols from cocoa or black tea. The presence of milk proteins bound to flavonoids was shown to weaken the flavonoid antioxidant capacity in vitro (12), and milk consumption has been shown to blunt the vascular benefits of tea flavonoids in healthy volunteers (13). Some carbohydrate-rich foods may increase the deglycosylation and absorption of flavonoids by stimulating gastrointestinal motility, mucosal blood flow, and colonic fermentation. Conversely, dietary flavonoids have been shown to interfere with carbohydrate digestion and absorption (see Biological Activities).
In the large intestine, gut microbial enzymes transform flavonoids through deglycosylation, ring fission, dehydroxylation, demethylation, etc. into metabolites that can then be absorbed or excreted (9, 14). The diversity and activity of colonic bacteria, which are partly dependent on a person’s dietary habits, will determine which metabolites can be produced from ingested flavonoids (15, 16). The composition of the colonic microbiota can therefore affect the metabolic fate and bioavailability of dietary flavonoids (17).
Flavonoids are recognized as xenobiotics by the body such that they undergo extensive modifications first in the intestinal mucosa and then in the liver.
Depending on their structural characteristics, flavonoids can be rapidly transformed by phase II detoxification enzymes to form methylated, glucuronidated, and/or sulfated metabolites (2). This metabolic pathway increases the solubility of phenolic aglycones and facilitates their excretion in the bile and urine (11). Free (unconjugated) aglycones are generally absent from the bloodstream, with the possible exception of trace levels of catechins (17). Catechol-O-methyltransferase (COMT) is the detoxifying enzyme responsible for the methylation of the hydroxyl groups of flavonoids, producing O-methylated flavonoids. A single nucleotide polymorphism (SNP) in the gene for COMT — known as SNP rs4680 G>A — causes a valine-to-methionine substitution in the sequence of the enzyme. Individuals with the A/A genotype have a form of the enzyme that is three- to four-fold less active than the wild-type variant in G/G genotype carriers (18). It has been suggested that subjects who are less efficient at eliminating green tea flavonoids may be more likely to benefit from their consumption (19).
Flavonoid conjugates are excreted via the action of efflux transporters from the ATP-binding cassette (ABC) family, including P-glycoprotein, MRPs (multidrug resistance proteins), or BCRPs (breast cancer-resistant proteins). Depending on their physicochemical properties, some flavonoids may interfere with the activity of ABC transporters (20). This implies that flavonoids can affect their own bioavailability, as well as that of other substrates of these transporters (e.g., pharmacological drugs) (see Drug interactions).
Flavonoid bioavailability may be inversely related to their binding affinity to plasma proteins (21). Greater binding affinity to plasma proteins (and thus, possibly, lower flavonoid bioavailability) has been linked to structural characteristics, such as methylation and galloylation. On the contrary, glycosylation reduced binding affinity to plasma proteins, suggesting that aglycones might have a limited bioavailability compared to glycosylated flavonoids. While glucuronidation is thought to facilitate the excretion of flavonoids from the body, glucuronides show little affinity to plasma proteins and might thus be able to diffuse to target tissues where deglucuronidation can take place (8).
In general, the bioavailability of flavonoids is low due to limited absorption, extensive metabolism, and rapid excretion. Isoflavones are thought to be the most bioavailable of all flavonoid subclasses, while anthocyanins and galloylated catechins are very poorly absorbed (8, 22). Yet, given the wide variability in structures within subclasses, it is difficult to generalize the absorbability and bioavailability of flavonoids based only on their structural classification. In addition, when evaluating the data from flavonoid research in cultured cells, it is important to consider whether the flavonoid concentrations and metabolites used are physiologically relevant (23). In humans, peak plasma concentrations of soy isoflavones and citrus flavanones have not been found to exceed 10 micromoles/liter (μM) after oral consumption. Peak plasma concentrations measured after the consumption of anthocyanins, flavan-3-ols, and flavonols (including those from tea) are generally lower than 1 μM (2). A recent quantitative analysis of 88 polyphenolic metabolites (not limited to flavonoids) identified in human blood and urine found median peak concentrations of 0.9 μM and 3.2 μM after food intake and oral supplementation, respectively (4).
Flavonoids are effective scavengers of free radicals in the test tube (in vitro) (24, 25). However, even with very high flavonoid intakes, plasma and intracellular flavonoid concentrations in humans are likely to be 100 to 1,000 times lower than concentrations of other antioxidants, such as ascorbate (vitamin C), uric acid, and glutathione. Moreover, most circulating flavonoids are actually flavonoid metabolites, some of which have lower antioxidant activity than the parent flavonoid (5). For these reasons, the relative contribution of dietary flavonoids to plasma and tissue antioxidant function in vivo is likely to be very small or negligible (26-28).
Metal ions, such as iron and copper, can catalyze the production of free radicals. The ability of flavonoids to chelate (bind) metal ions appears to contribute to their antioxidant activity in vitro (29, 30). In living organisms, most iron and copper are bound to proteins, limiting their participation in reactions that produce free radicals. Although the metal-chelating activities of flavonoids may be beneficial in pathological conditions of iron or copper excess, it is not known whether flavonoids or their metabolites function as effective metal chelators in vivo (26).
Cells are capable of responding to a variety of different stresses or signals by increasing or decreasing the availability of specific proteins. The complex cascades of events that lead to changes in the expression of specific genes are known as cell-signaling pathways or signal transduction pathways. These pathways regulate numerous cell processes, such as proliferation, differentiation, inflammatory responses, apoptosis (programmed cell death), and survival. Although it was initially hypothesized that the biological effects of flavonoids would be related to their antioxidant activity, available evidence from cell culture experiments suggests that many of the effects of flavonoids, including antiinflammatory, antidiabetic, anticancer, and neuroprotective activities, are related to their ability to modulate cell-signaling pathways (27). Intracellular concentrations of flavonoids required to affect cellular signaling are considerably lower than those required to affect cellular antioxidant capacity. Flavonoid metabolites may retain their ability to interact with cell-signaling proteins even if their antioxidant activity is diminished (31, 32).
Effective signal transduction requires proteins known as kinases that catalyze the phosphorylation of target proteins, which become either activated or inhibited. Cascades involving specific phosphorylations or dephosphorylations of signal transduction proteins ultimately affect the activity of transcription factors — proteins that bind to specific response elements on DNA and promote or prevent the transcription of target genes. Results of numerous studies in cell culture suggest that flavonoids may affect chronic disease by selectively inhibiting kinases (27, 33). Cell growth and proliferation are also regulated by growth factors that initiate cell-signaling cascades by binding to specific receptors in cell membranes. Flavonoids may alter growth factor signaling by inhibiting receptor phosphorylation or blocking receptor binding by growth factors (34).
Each flavonoid subclass contains many types of chemicals with varying biological activities (and potential health benefits) such that the activity of a specific flavonoid cannot easily be generalized. Some examples of major biological activities of flavonoids are highlighted below.
Flavonoids have been shown to (1) reduce inflammation by suppressing the expression of pro-inflammatory mediators (35-37); (2) down-regulate the expression of vascular cell adhesion molecules, which contribute to the recruitment of inflammatory white blood cells from the blood to the arterial wall (38, 39); (3) increase the production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS), thus improving vascular endothelial function (40); (4) inhibit angiotensin-converting enzyme, thus inducing vascular relaxation (41); (5) inhibit platelet aggregation (42); and (6) oppose smooth muscle cell proliferation and migration occurring during atherogenesis (43).
Flavonoids have been found to interfere with the digestion, absorption, and metabolism of carbohydrates (reviewed in 44). Each subclass of flavonoids has also demonstrated anti-diabetic properties, including (1) improving insulin secretion and viability of pancreatic β-cells under glucotoxic or pro-inflammatory conditions, (2) increasing insulin-stimulated glucose uptake by target cells, (3) protecting muscle cells against fatty acid-induced insulin resistance, and (4) reducing hyperglycemia and improving glucose tolerance in animal models of obesity and/or type 2 diabetes mellitus (45).
Flavonoids have been found to (1) scavenge free radicals that can damage macromolecules, including DNA (46, 47); (2) interfere with biotransformation enzymes and efflux transporters, possibly preventing the activation of procarcinogenic chemicals and promoting their excretion from the body (48, 49); (3) regulate proliferation, DNA repair, or activation of pathways leading to apoptosis (programmed cell death) in case of irreversible DNA damage (50); and (4) inhibit tumor invasion and angiogenesis (51, 52).
Flavonoids are thought to (1) promote neurogenesis, synaptic growth, and neuron survival in the learning and memory-related brain regions (e.g., hippocampus) by stimulating the production of neurotrophins like BDNF; (2) protect hippocampal cells and striatal dopaminergic cells from cytotoxic molecules (pro-inflammatory mediators and ROS) released by abnormally activated microglia and hypertrophic astrocytes in neurodegenerative disorders; (3) reduce neuroinflammation by inhibiting the generation of pro-inflammatory cytokines, lipid mediators, and reactive oxygen species by astrocytes and microglial cells; (4) stimulate the production of nitric oxide (NO), which improves endothelial function, increases cerebral blood flow, and protects artery walls against the buildup of atherosclerotic plaques (reviewed in 53, 54).
Several prospective cohort studies conducted in the US and Europe have examined the relationship between some measure of dietary flavonoid intake and cardiovascular disease (CVD) or mortality. A recent meta-analysis of 14 prospective studies published between 1996 and 2012 reported that higher intakes in each flavonoid subclass were significantly associated with a reduced risk of cardiovascular events (55). Top versus bottom quantiles of intake for each of the flavonoid subclasses were associated with an approximate 10% reduction in the risk of CVD. Another meta-analysis of eight prospective studies found a 14% reduced risk of stroke with the highest versus lowest quintile of flavonol intakes (56). However, several serious limitations highlighted in a recent publication by Jacques et al. suggested caution when interpreting these results (57). In particular, most of the prospective studies in these meta-analyses did not include all flavonoid subclasses nor calculate intakes using the latest and more complete versions of the USDA databases for the flavonoid content of foods (58-60). Another major concern is the lack of adjustment regarding the overall quality of the diet. Consumers with higher flavonoid intakes are likely to have a greater consumption of fruit and vegetables and overall healthier diets than those with poor flavonoid intakes. Additionally, none of the studies excluded potential bias due to constituents of flavonoid-rich foods that are known to either lower (e.g., other phytochemicals, vitamins, dietary fiber) or increase (e.g., sodium, saturated fat) the risk of cardiovascular events (discussed in 57).
In the Framingham Offspring Cohort study that followed 2,880 adults for a mean of 14.9 years, consumption of all flavonoid subclasses except flavones and flavanones was inversely associated with CVD (57). Yet, adjusting for confounding factors, including fruit and vegetable intake and overall diet quality, attenuated these relationships such that they were no longer statistically significant. An analysis of a larger prospective study of the EPIC-Norfolk cohort (24,885 participants) that considered confounding by many dietary factors (vitamin C, dietary fiber, fat, saturated fat, potassium, sodium, and alcohol) found no significant association between flavan-3-ol intake and CVD-related or all-cause mortality (61).
A number of large prospective studies and small-scale, randomized controlled trials have investigated the effects of flavonoids on established biomarkers of CVD, including those involved in oxidative stress, inflammation, abnormal blood lipid profile, endothelial dysfunction, and hypertension; some of these studies are highlighted below.
In a cross-sectional analysis of the Framingham Offspring Cohort study, the highest versus lowest intake of anthocyanins (≤3.5 mg/day versus ≥23.5 mg/day) was associated with lower concentrations of acute-phase reactant proteins (-100%), pro-inflammatory cytokines (-75%), and markers of oxidative stress (-52%), even after adjustment for confounding variables (62). Interestingly, a food-based analysis revealed that intakes of foods rich in anthocyanins, e.g., apples, red wine, and strawberries, were also inversely associated with an overall inflammation score based on 12 different biomarkers. Higher intakes of polymeric flavan-3-ols (i.e., theaflavins, thearubigins, and proanthocyanidins) were correlated with lower concentrations of pro-inflammatory cytokines and biomarkers of oxidative stress. Intake levels of total flavonoids and flavan-3-ol monomers (i.e., catechins) were inversely associated with concentrations of the biomarkers of oxidative stress. Although tea is a major source of flavan-3-ols, tea consumption was not correlated with the composite inflammation score or any components of this score in this study (62).
Cocoa is another source of flavan-3-ols, in particular (-)-epicatechin and procyanidins, that may provide cardiovascular benefits (63). Indeed, a recent randomized, double-blind, placebo-controlled study in 100 healthy adults (ages, 35-60 years) suggested that short-term benefits of cocoa flavan-3-ol consumption on cardiovascular health, including improvements in lipoprotein profile (i.e., higher HDL-cholesterol and lower total and LDL-cholesterol) and blood pressure, could be extrapolated to predict a 20%-30% reduced 10-year risk of CVD and CVD-related mortality (64).
An increasing number of trials in which participants were fed with berries (65-67) or juices (68) rich in anthocyanins or with purified anthocyanins (69) also reported reduced levels of inflammatory markers and/or improved antioxidant status, decreased LDL-cholesterol, improved insulin sensitivity, and lowered blood pressure (reviewed in 70). In a randomized, double-blind, placebo-controlled study in 150 individuals with hypercholesterolemia, supplementation with a purified anthocyanin mixture (320 mg/day) for 24 weeks reduced circulating markers of inflammation, including C-reactive protein (CRP), interleukin-1β (IL-1β), and soluble vascular adhesion molecule-1 (sVCAM-1) (71). Supplementation of dyslipidemic patients for 12 or 24 weeks with a mixture of 17 anthocyanins improved cholesterol clearance via the HDL-mediated reverse cholesterol transport from extra-hepatic tissues back to the liver and lowered LDL-cholesterol compared to a placebo in two randomized controlled trials (72, 73). However, a 12-week, randomized, double-blind, placebo-controlled study in 52 healthy postmenopausal women found that daily consumption of 500 mg of elderberry anthocyanins (as cyanidin-3-glucoside) had no effect on inflammation markers, markers of vascular health, lipid profile, and glycemia; all of these measures were in normal range of concentrations at baseline (74). Whether exposure to high-dose anthocyanins could lower the risk of CVD in subjects with established CVD risk factors and/or help maintain cardiovascular health in apparently healthy individuals remains to be confirmed.
The vascular endothelial cells that line the inner surface of all blood vessels synthesize an enzyme, endothelial nitric oxide synthase (eNOS), whose function is essential to normal vascular physiology. Specifically, eNOS produces nitric oxide (NO), a compound that regulates vascular tone and blood flow by promoting the relaxation (vasodilation) of all types of blood vessels, including arteries (75). NO also regulates vascular homeostasis and protects the integrity of the endothelium by inhibiting vascular inflammation, leukocyte adhesion, platelet adhesion and aggregation, and proliferation of vascular smooth muscle cells (76). In the presence of cardiovascular risk factors (e.g., hypertension, hypercholesterolemia, hyperglycemia), early alterations in the structure and function of the vascular endothelium are associated with the loss of normal NO-mediated endothelium-dependent vasodilation. Endothelial dysfunction results in widespread vasoconstriction and coagulation abnormalities and is considered to be an early step in the development of atherosclerosis. Measures of brachial flow-mediated dilation (FMD), a surrogate marker of endothelial function, have been found to be inversely associated with risk of future cardiovascular events (77).
Preclinical studies have demonstrated the benefits of berry fruits, extracts, or purified anthocyanins on vascular function. Anthocyanin supplementation to diabetic mice was found to improve diabetes-induced vascular dysfunction by promoting NO-mediated endothelium-dependent vasodilation through the upregulation of adipocyte-derived adiponectin (78). Supplementation with purified anthocyanins (320 mg/day for 12 weeks) also increased serum adiponectin concentrations and improved FMD in 58 individuals with type 2 diabetes (78). In a randomized trial of 150 participants with hypercholesterolemia, supplemental anthocyanins increased FMD values by 28.4% compared to 2.2% in the placebo group (79).
Several small-scale, intervention studies have also examined the effect of flavan-3-ol-rich food and beverages, including tea, red wine, purple grape juice, cocoa, and chocolate, on endothelium-dependent vasodilation. A meta-analysis of nine intervention studies in a total of 213 participants estimated that the acute ingestion of 2 to 3 cups of tea (500 mL) — containing about 248 mg of flavonoids in green tea and 415 mg in black tea — significantly increased brachial FMD (see also the article on Tea) (80). Another meta-analysis of 18 randomized controlled studies found that acute (2 h post-ingestion) and chronic (≤18 months) consumption of flavan-3-ol-rich cocoa beverages and chocolate bars significantly increased FMD in participants (81). A small 15-day, cross-over intervention study in hypertensive individuals with endothelial dysfunction found that 100 g/day of flavan-3-ol-rich dark chocolate, but not 90 g/day of flavan-3-ol-free white chocolate, could restore FMD values almost to normal levels (82). Also, using a similar protocol, the authors showed that dark chocolate intake blunted acute endothelial dysfunction-induced by a glucose load challenge in 12 healthy volunteers (83). Other benefits of dark chocolate consumption included reductions in arterial stiffness (measured through pulse wave analysis) and serum concentrations of markers of oxidative stress and vasoconstriction (8-isoprostaglandin F2α and endothelin-1). A randomized controlled trial in overweight and obese participants also reported that the daily consumption of a high-flavan-3-ol cocoa drink (902 mg/day of flavan-3-ols), but not that of a cocoa drink low in flavan-3-ols (38 mg/day), resulted in a sustained increase in FMD during the 12-week study (84). A more recent four-week, randomized, double-blind, cross-over, controlled study in healthy overweight or obese adults found that the consumption of 22 g/day of natural cocoa (in the form of dark chocolate bar and cocoa drink; 814 mg/day of flavan-3-ols) increased arterial diameter and blood flow and lowered peripheral arterial stiffness, but there was no change in FMD (85). Another recent clinical trial found improvements in endothelium-dependent vasodilation in response to acute consumption of one bar (40 g) of dark chocolate (containing 10.8 mg of (+)-catechins and 36 mg of (-)-epicatechins) and daily consumption of two bars (80 g) for up to four weeks in 20 individuals with chronic heart failure (86). Oral administration of pure flavan-3-ol (-)-epicatechin to healthy volunteers showed NO-dependent vasodilatory effects similar to those observed following flavan-3-ol-rich cocoa ingestion (87). Administration of (-)-epicatechin also improved acetylcholine-induced endothelial-dependent vasodilation of thoracic aorta rings from rats with salt-induced hypertension (88).
Endothelial nitric oxide production also inhibits the adhesion and aggregation of platelets, one of the first steps in atherosclerosis and blood clot formation (76). A number of clinical trials that examined the potential for high flavonoid intakes to decrease various measures of platelet function outside of the body (ex vivo) have reported mixed results. A recent systematic review of these intervention studies suggested that consumption of flavan-3-ol-rich cocoa and grape seed extract was generally found to improve platelet function by inhibiting platelet adhesion, activation, and aggregation (89). Interestingly, in a cross-over, controlled study, the acute consumption of a flavan-3-ol-rich cocoa beverage (897 mg of total (-)-EC and procyanidins) exhibited additive anti-platelet effects to aspirin (81 mg) in healthy volunteers (90). In contrast, the results of interventions using apigenin-rich soup, quercetin-rich supplements or onion soups, isoflavone-rich soy protein isolates, black tea, wines, berries, or grape juices have given inconsistent results (reviewed in 89).
A meta-analysis of 20 short-term, randomized controlled trials, including a total of 856 mainly healthy participants, found that consumption of flavan-3-ol-rich dark chocolate and cocoa products significantly reduced systolic blood pressure by 2.77 mm Hg and diastolic blood pressure by 2.20 mm Hg. However, heterogeneity across studies was high, and risk of bias was significant (91). A greater blood pressure-reducing effect was observed in a subanalysis of studies using flavan-3-ol-free rather than flavan-3-ol-low control groups (91). Another meta-analysis of 22 trials (highly heterogeneous) found reductions in diastolic blood pressure (-1.60 mm Hg) and mean arterial pressure (-1.64 mm Hg) with chocolate or cocoa intake but no change in systolic blood pressure (81). Additionally, green tea flavan-3-ols have been shown to lower blood pressure especially in (pre-) hypertensive subjects. A pooled analysis of 13 randomized controlled trials in 1,040 subjects found a 2.05 mm Hg reduction in systolic blood pressure and a 1.71 mm Hg reduction in diastolic blood pressure with green tea consumption for at least three weeks (92). The inhibition of angiotensin-converting enzyme (ACE), a key regulator of arterial blood pressure, may partly explain how flavan-3-ol-rich food and beverages might exert blood pressure-lowering effects (93).
Some intervention trials have also examined the effect of the flavonol quercetin on blood pressure in human subjects. In a randomized, double-blind, cross-over, placebo-controlled trial in 96 participants diagnosed with metabolic disorders, supplementation with 150 mg/day of quercetin aglycone significantly reduced systolic blood pressure by 2.6 mm Hg without affecting diastolic blood pressure and other cardiometabolic markers (94). Similar results were found with 730 mg/day of quercetin in hypertensive individuals (95) and with 500 mg/day of quercetin in women with type 2 diabetes mellitus (96). In a recent six-week, cross-over, randomized, double-blind, placebo-controlled trial, daily ingestion of 162 mg of quercetin decreased 24 h-ambulatory blood pressure — but not systolic blood pressure in the resting state — in hypertensive but not in pre-hypertensive participants (97). There was no change in biomarkers of lipid metabolism, inflammation, oxidative stress, or endothelial function, including total, HDL-, LDL-cholesterol, serum CRP, soluble adhesion molecules, plasma oxidized LDL, urinary 8-isoprostaglandin F2α, serum endothelin-1, serum ACE, and plasma endogenous NOS inhibitor.
Additional trials may help establish whether the blood pressure-lowering effect of some flavonoids could be translated into long-term benefits for cardiovascular health.
The association between flavonoid consumption and risk for type 2 diabetes mellitus has been examined in a recent European, multicenter, nested case-control study — the "EPIC-InterAct" project — that included 16,835 diabetes-free participants and 12,043 diabetics. In this study, participants in the highest quintile of total flavonoid intake (>608.1 mg/day) had a 10% lower risk of diabetes than those in the lowest quintile (<178.2 mg/day) (98). Specifically, the risk of diabetes was inversely correlated with the intake of flavan-3-ols (monomers and dimers only) and flavonols (98, 99). Recent meta-analyses of randomized controlled trials have examined the possible health effects of green tea flavan-3-ol monomers (catechins) on glucose metabolism and have provided conflicting results. A meta-analysis of seven trials in pre-diabetic and diabetic patients found no effect of green tea or green tea extracts on fasting plasma glucose, fasting serum insulin, or measures of glycemic control (glycated hemoglobin, HbA1c) and insulin sensitivity (HOMA-IR) (100). Conversely, another meta-analysis of 17 trials in pre-diabetic, diabetic, or overweight/obese subjects found that administration of green tea extracts for 4 to 16 weeks improved fasting plasma glucose and HbA1c level (101). The effect on fasting glucose was observed only with high doses of catechins (≥457 mg/day) and when the confounding effect of caffeine was removed. Finally, a third meta-analysis of 25 trials found that ingestion of green tea extracts for at least two weeks could lower fasting blood glucose in both the presence or absence of caffeine (102).
Dark chocolate is another good source of flavan-3-ols such that the effects of cocoa flavan-3-ols have been examined in individuals at-risk or with established type 2 diabetes. In a 15-day, cross-over, randomized controlled study, the daily consumption of 100 g of dark chocolate bars containing 110.9 mg of (-)-EC and 36.1 mg of (+)-C significantly improved measures of pancreatic β-cell function and insulin sensitivity, along with cardiometabolic markers in glucose-intolerant and hypertensive subjects (103). Daily supplementation with flavonoid-enriched chocolate containing 850 mg of flavan-3-ols and 100 mg of isoflavones for one year significantly improved insulin sensitivity and reduced a predicted risk of coronary heart disease (CHD) at 10 years in 93 postmenopausal women treated for type 2 diabetes (104).
The EPIC-InterAct study did not find any association between dietary anthocyanin intake and risk of diabetes (98, 99). Yet, a 10-fold increase in anthocyanin consumption was correlated with a 15% lower risk of diabetes in the pooled analysis of three large US prospective cohorts (120,003 participants) (105). Of note, this pooled analysis also reported a moderately higher risk of diabetes (+6%) in individuals in the highest versus lowest quintiles of flavone and flavanone intakes. Moreover, the consumption of berries, rich in anthocyanins, has been shown to trigger favorable glycemic responses in type 2 diabetics (reviewed in 70). In recent intervention studies, anthocyanins demonstrated beneficial effects on metabolic abnormalities in patients at-risk or diagnosed with diabetes. In an eight-week, randomized, double-blind, placebo-controlled trial in 38 healthy overweight and obese subjects, the consumption of 2 g/day of grape polyphenols rich in proanthocyanidins and anthocyanins prevented increases in oxidative stress and insulin resistance induced by a six-day, high-fructose challenge (106). Another six-week randomized trial in individuals with diabetes showed that daily supplementation with Cornelian cherry (Cornus mas) extracts containing 600 mg of anthocyanins significantly lowered serum levels of HbA1c and triglycerides and increased serum insulin concentrations (107). The administration of 320 mg/day of anthocyanins for 24 weeks also improved serum lipid and lipoprotein profile, decreased markers of oxidative stress and inflammation, elevated antioxidant capacity, and reduced insulin resistance compared to a placebo in patients with diabetes (108). Further, supplemental anthocyanins up-regulated adiponectin expression and improved nitric oxide-mediated endothelium-dependent vasodilation within 12 weeks of treatment (see also Cardiovascular disease) (78).
These promising findings warrant additional randomized controlled trials to confirm preventive and/or therapeutic benefits of (cocoa) flavan-3-ols and anthocyanins in type 2 diabetes.
Although various flavonoids have been found to inhibit the development of chemically-induced cancers in animal models of lung (109), oral (110), esophageal (111), gastric (112), colon (113), skin (114), prostate (115, 116), and mammary cancer (117), observational studies do not provide convincing evidence that high intakes of dietary flavonoids are associated with substantial reductions in human cancer risk (reviewed in 118). A meta-analysis of 13 case-control and 10 prospective cohort studies found little-to-no evidence to support a preventive role of dietary flavonoid intake in gastric and colorectal cancer (119). In addition, a recently published analysis of two large prospective studies (the Health Professionals Follow-up Study [HPFS] and the Nurses’ Health Study [NHS]) — using the most up-to-date flavonoid food composition databases — found no association between the risk of colorectal cancer and intakes of each subclass of flavonoids or flavonoid-rich foods (tea, blueberries, oranges) (120). A meta-analysis of 19 case-control studies and 15 cohort studies found that total flavonoid intake and intakes of specific flavonoid subclasses (i.e., flavonols, flavones, flavanones) were inversely correlated with the risk of smoking-sensitive cancers of the aerodigestive tract (mouth, pharynx, larynx, esophagus, and stomach) in smokers but not in nonsmokers (121). The risk of lung cancer was not significantly associated with high flavonoid intakes (121), although an earlier meta-analysis of eight prospective studies (with substantial heterogeneity across them) suggested a protective role of flavonoids against lung cancer in smokers only (122). Further, a prospective analysis of over 45,000 postmenopausal women from the Multiethnic Cohort Study found a reduced risk of endometrial cancer with the highest intakes of total isoflavones, daidzein, and genistein (123). Additionally, limited evidence from observational studies suggests no relationship between total flavonoid intake and ovarian cancer (124-127). To date, there is little evidence that flavonoid-rich diets might protect against various cancers, but larger prospective cohort studies are needed to address the association.
Because isoflavones are phytoestrogens, it is thought that they may interfere with the synthesis and activity of endogenous hormones, eventually influencing hormone-dependent signaling pathways and protecting against breast and prostate cancers (128). A meta-analysis of 14 observational studies that examined breast cancer incidence in 369,934 women found an overall 11% reduced risk of breast cancer with the highest versus lowest intake of soy isoflavones (129). Subgroup analyses revealed a 24% lower risk of cancer in Asian but not in European or US women, and the risk was 22% lower in postmenopausal but not lower in premenopausal women. In addition to the ethnicity and menopausal status, polymorphisms for hormone receptors (130) and phase I biotransformation enzymes (131) have been found to modify the association between isoflavone intake and breast cancer. Another recent meta-analysis of 12 observational studies (six prospective cohort studies, one nested case-control study, and five case-control studies) investigated the chemopreventive effects of flavonoids (except isoflavones) (132). The results suggested that intakes of flavonols and flavones may also be inversely associated with the risk of breast cancer. Further, a pooled analysis of four case-control studies that stratified by menopausal status showed inverse associations between breast cancer and intakes of flavonols, flavones, or flavan-3-ols in postmenopausal women only. Finally, a meta-analysis of four prospective cohort studies found an overall 16% reduced risk of breast cancer recurrence in women with high versus low isoflavone intakes (129).
A meta-analysis of 13 observational studies also suggested an inverse relationship between prostate cancer risk and consumption of soy products, especially tofu (133). Yet, further analyses supported a protective role of soy food based only on case-control studies, which have inherent flaws such that associations may often be overestimated or underestimated. In a recent 12-month, multicenter, randomized, double-blind, placebo-controlled phase II clinical trial in 158 Japanese men (aged ≥50 years) with elevated risk of prostate cancer, oral isoflavone (60 mg/day) resulted in a significant decrease in prostate cancer incidence in participants aged 65 years and older (134). In this study, no changes were reported in sex hormone concentrations in blood, suggesting that isoflavones may reduce prostate cancer incidence without interfering with hormone-dependent pathways.
Additional investigations will be necessary to determine whether supplementation with specific flavonoids could benefit cancer prevention or treatment.
For more information on flavonoid-rich foods and cancer, see articles on Fruit and Vegetables, Legumes, and Tea.
Inflammation, oxidative stress, and transition metal accumulation appear to play a role in the pathology of several neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease (135). Therefore, the various properties of flavonoids, including their role in protecting vascular health, could have beneficial effects on the brain, possibly in the protection against cerebrovascular disorders, cognitive impairments, and subsequent stroke and dementias. Dietary flavonoids and/or their metabolites have been shown to cross the blood-brain barrier (54) and exert preventive effects towards cognitive impairments in animal models of normal and pathological aging (53).
The cross-sectional data analysis of 2,031 participants (ages, 70-74 years) from the Hordaland Health Study in Norway indicated that, when compared to non-consumers, consumers of flavonoid-rich chocolate, tea, and wine had better global cognitive function, assessed by a battery of six cognitive tests (136). The risk of poor performance in all tests was estimated to be 60 to 74% lower in consumers of all three flavonoid-rich foods compared to non-consumers. An early prospective cohort study in 1,367 older French men and women (aged ≥65 years; free from dementia at baseline) found that those with the lowest flavonoid intakes (<11.5 mg/day) had a 50% higher risk of developing dementia over the next five years than those with higher intakes (137). In addition, those with higher dietary flavonoid intakes at baseline experienced significantly less age-related cognitive decline over a 10-year period than those with the lowest flavonoid intakes (138).
The effect of cocoa flavan-3-ols have been investigated in an eight-week, randomized, double-blind trial — the Cognitive, Cocoa, and Aging (CoCoA) study — in 90 individuals (ages, 64-82 years) with mild cognitive impairments (MCI); participants were given dairy-based cocoa drinks with either high (993 mg/day) or low (48 mg/day) levels of flavan-3-ols (139). The daily consumption of the cocoa drink high in flavan-3-ols improved some, but not all, measures of cognitive process speed and flexibility and verbal fluency compared to baseline test scores and scores following low flavan-3-ol drink consumption. A composite test score reflecting overall cognitive performance was found to be significantly greater in those given cocoa drinks high rather than low in flavan-3-ols. The study also reported reductions in cardiovascular risk markers (i.e., systolic and diastolic blood pressure, total and LDL-cholesterol, insulin resistance), and these changes were proposed to partly contribute to ameliorate cognitive performance in those who consumed the flavan-3-ol-rich cocoa drink (139). The data could be replicated in cognitively healthy older people (ages, 61-85 years), suggesting that cocoa flavan-3-ols might enhance some aspects of cognitive function during healthy aging (140). Interestingly, a two-week, randomized, double-blind, controlled study has reported an increase in blood flow velocity in the middle cerebral artery of 21 healthy subjects (mean age, 72 years) following the daily intake of a flavan-3-ol-rich cocoa drink (900 mg/day of flavan-3-ols) (141). Because cerebral blood flow is correlated with cognitive function in humans, these preliminary data suggest that cocoa flavan-3-ol consumption could exert a protective effect against dementia (54).
Yet, in other randomized controlled trials (142-144), the lack of an effect of cocoa flavan-3-ols on blood pressure, cerebral blood flow, mental fatigue, and cognitive performance in healthy young and old adults suggested that benefits may only be seen in very demanding cognitive exercises (145).
Some randomized controlled studies also reported improvements in measures of cognitive function in healthy and cognitively impaired subjects with other flavonoid subclasses, including anthocyanins (146), flavanones (147, 148), and isoflavones (149, 150). Although some flavonoids and flavonoid-rich foods may enhance cognitive function in the aging brain, it is not yet clear whether their consumption could lower the risk of cognitive impairments and dementia in humans.
For more detailed information on flavan-3-ol-rich tea and cognitive function, see the article on Tea.
Recent data analyses of the National Health and Nutrition Examination Survey (NHANES) estimated flavonoid intakes in US adults (aged ≥19 years) average between 200 and 250 mg/day, with 80% being flavan-3-ols, 8% for flavonols, 6% for flavanones, 5% for anthocyanidins, and ≤1% for isoflavones and flavones (151, 152). The main dietary sources of flavonoids include tea, citrus fruit, citrus fruit juices, berries, red wine, apples, and legumes. Individual flavonoid intakes may vary considerably depending on whether tea, red wine, soy products, or fruit and vegetables are commonly consumed (reviewed in 2). Information on the flavonoid content of some flavonoid-rich foods is presented in Tables 2-8. These values should be considered approximate since a number of factors may affect the flavonoid content of foods, including agricultural practices, environmental conditions, ripening, storage, and food processing. For additional information about the flavonoid content of food, the USDA provides databases for the content of selected foods in flavonoids (60) and proanthocyanidins (58). For more information on the isoflavone content of soy foods, see the article on Soy Isoflavones or the USDA database for the isoflavone content of selected foods (59).
Bilberry, elderberry, black currant, blueberry, red grape, and mixed berry extracts that are rich in anthocyanins are available as dietary supplements without a prescription in the US. The anthocyanin content of these products may vary considerably. Standardized extracts that list the amount of anthocyanins per dose are available.
Numerous tea extracts are available in the US as dietary supplements and may be labeled as tea catechins or tea polyphenols. Green tea extracts are the most commonly marketed, but black and oolong tea extracts are also available. Green tea extracts generally have higher levels of catechins (flavan-3-ol monomers), while black tea extracts are richer in theaflavins and thearubigins (tea flavan-3-ol dimers and polymers, respectively). Oolong tea extracts fall somewhere in between green and black tea extracts with respect to their flavan-3-ol content. Some tea extracts contain caffeine, while others are decaffeinated. Flavan-3-ol and caffeine content vary considerably among different products, so it is important to check the label or consult the manufacturer to determine the amounts of flavan-3-ols and caffeine that would be consumed daily with each supplement (for more information on tea flavan-3-ols, see the article on Tea).
Citrus bioflavonoid supplements may contain glycosides of hesperetin (hesperidin), naringenin (naringin), and eriodictyol (eriocitrin). Hesperidin is also available in hesperidin-complex supplements, with daily doses from 500 mg to 2 g (153).
The peels and tissues of citrus fruit (e.g., oranges, tangerines, and clementines) are rich in polymethoxylated flavones: tangeretin, nobiletin, and sinensetin (2). Although dietary intakes of these naturally occurring flavones are generally low, they are often present in citrus bioflavonoid complex supplements. Several dietary supplements may also contain various amounts of baicalein (aglycone) and/or baicalin (glycoside). Some tea preparations may also include baicalein-7-glucuronide (153).
The flavonol aglycone, quercetin, and its glycoside rutin are available as dietary supplements without a prescription in the US. Other names for rutin include rutoside, quercetin-3-rutinoside, and sophorin (153). Citrus bioflavonoid supplements may also contain quercetin or rutin.
A 50-mg soy isoflavone supplement usually includes glycosides of the isoflavones: genistein (genistin; 25 mg), daidzein (daidzin; 19 mg), and glycitein (glycitin; about 6 mg). Smaller amounts of daidzein, genistein, and formononetin are also found in biochanin A-containing supplements (derived from red clover) (153).
No adverse effects have been associated with high dietary intakes of flavonoids from plant-based food. This lack of adverse effects may be explained by the relatively low bioavailability and rapid metabolism and elimination of most flavonoids.
Oral supplementation with quercetin glycosides at doses ranging between 3 mg/day-1,000 mg/day for up to three months has not resulted in significant adverse effects in clinical studies (reviewed in 154). A randomized, placebo-controlled study in 30 patients with chronic prostatitis reported one case of headache and another of tingling of the extremities associated with supplemental quercetin (1,000 mg/day for one month); both issues resolved after the study ended (155). In a phase I clinical trial in cancer patients unresponsive to standard treatments, administration of quercetin via intravenous infusion resulted in symptoms of nausea, vomiting, sweating, flushing, and dyspnea (difficulty breathing) at doses ≥10.5 mg/kg body weight (~756 mg of quercetin for a 70 kg individual) (156). Higher doses up to 51.3 mg/kg body weight (~3,591 mg of quercetin) were associated with renal (kidney) toxicity, yet without evidence of nephritis, infection, or obstructive uropathy (reviewed in 154).
In a recent randomized, double-blind, controlled study in healthy adults, the daily intake of 2 g of cocoa flavan-3-ols for 12 weeks was found to be well tolerated with no adverse side effects (157).
In clinical trials employing caffeinated green tea extracts, cancer patients who took 6 g/day in three to six divided doses reported mild-to-moderate gastrointestinal side effects, including nausea, vomiting, abdominal pain, and diarrhea (158, 159). Central nervous system symptoms, including agitation, restlessness, insomnia, tremors, dizziness, and confusion, have also been reported. In one case, confusion was severe enough to require hospitalization (158). In a systematic review published in 2008, the US Pharmacopeia (USP) Dietary Supplement Information Expert Committee identified 34 adverse event reports implicating the use of green tea extract products (containing 25%-97% of polyphenols) as the likely cause of liver damage (hepatotoxicity) in humans (160). In a four-week clinical trial that assessed the safety of decaffeinated green tea extracts (800 mg/day of EGCG) in healthy individuals, a few of the participants reported mild nausea, stomach upset, dizziness, or muscle pain (161). In the Minnesota Green Tea Trial (MGTT), 1,075 postmenopausal women were randomized to receive green tea extracts (1,315±116 mg/day of catechins; the equivalent of four 8-ounce mugs of brewed decaffeinated green tea) or a placebo for one year. The total number of adverse events and the number of serious adverse events were not different between the treatment and placebo groups (162). However, the use of green tea extracts was directly associated with abnormally high liver enzyme levels in 7 out of the 12 women who experienced serious adverse events. Also, the incidence of nausea was twice as high in the green tea arm as in the placebo group (162).
The safety of flavonoid supplements in pregnancy and lactation has not been established (153).
ATP-binding cassette (ABC) drug transporters, including P-glycoprotein, multidrug resistance protein (MRP), and breast cancer-resistant protein (BCRP), function as ATP-dependent efflux pumps that actively regulate the excretion of a number of drugs limiting their systemic bioavailability (8). ABC transporters are found throughout the body, yet they are especially important in organs with a barrier function like the intestines, the blood-brain barrier, blood-testis barrier, and the placenta, as well as in liver and kidneys (163). There is some evidence that the consumption of grapefruit juice inhibits the activity of P-glycoprotein (164). Genistein, biochanin A, quercetin, naringenin, hesperetin, green tea flavan-3-ol (-)-CG, (-)-ECG, and (-)-EGCG, and others have been found to inhibit the efflux activity of P-glycoprotein in cultured cells and in animal models (163). Thus, very high or supplemental intakes of these flavonoids could potentially increase the toxicity of drugs that are substrates of P-glycoprotein, e.g., digoxin, antihypertensive agents, antiarrhythmic agents, chemotherapeutic (anticancer) agents, antifungal agents, HIV protease inhibitors, immunosuppressive agents, H2 receptor antagonists, some antibiotics, and others (reviewed in 165).
Many anthocyanins and anthocyanidins, as well as some flavones (apigenin, chrysin), isoflavones (biochanin A, genistein), flavonols (kaempferol), and flavanones (naringenin), have been identified as inhibitors of BRCP-mediated transport, theoretically affecting drugs like anticancer agents (mitoxantrone, topotecan, thyrosine kinase inhibitors), antibiotics (fluoroquinolones), β-blockers (prazosin), and antiarthritics (sulfasalazine). Finally, flavonols (quercetin, kaempferol, myricetin), flavanones (naringenin), flavones (apigenin, robinetin), and isoflavones (genistein) have been reported to inhibit MRP, potentially affecting MRP-mediated transport of many anticancer drugs, e.g., vincristin, etoposide, cisplatin, irinotecan, methotrexate, camptothecin, anthracyclines, vinca alkaloids (reviewed in 163).
High intakes of flavonoids from purple grape juice (500 mL/day) and dark chocolate (235 mg/day of flavan-3-ols) have been found to inhibit platelet aggregation in ex vivo assays (166-169). Theoretically, high intakes of flavonoids (e.g., from supplements) could increase the risk of bleeding when taken with anticoagulant drugs, such as warfarin (Coumadin), heparin, dalteparin (Fragmin), enoxaparin (Lovenox), and antiplatelet drugs, such as clopidogrel (Plavix), dipyridamole (Persantine), non-steroidal anti-inflammatory drugs (NSAIDs: diclofenac, ibuprofen, naproxen), aspirin, and others (170).
Cytochrome P450 (CYP) enzymes are phase I biotransformation enzymes involved in the metabolism of a broad range of compounds, from endogenous molecules to therapeutic agents. The most abundant CYP isoform in the liver and intestines is cytochrome P450 3A4 (CYP3A4); the CYP3A family catalyzes the metabolism of about one-half of all marketed drugs in the US and Canada (171). One grapefruit or as little as 200 mL (7 fluid ounces) of grapefruit juice have been found to irreversibly inhibit intestinal CYP3A4 (164). The most potent inhibitors of CYP3A4 in grapefruit are thought to be furanocoumarins, particularly dihydroxybergamottin, rather than flavonoids. All forms of the fruit — freshly squeezed juice, frozen concentrate, or whole fruit — can potentially affect the activity of CYP3A4. Some varieties of other citrus fruit (Seville oranges, limes, and pomelos) that contain furanocoumarins can also interfere with CYP3A4 activity.
Specifically, the inhibition of intestinal CYP3A4 by grapefruit consumption is known or predicted to increase the bioavailability and the risk of toxicity of more than 85 drugs. Because drugs with very low bioavailability are more likely to be toxic when CYP3A4 activity is inhibited, they are associated with a higher risk of overdose with grapefruit compared to drugs with high bioavailability. Some of the drugs with low bioavailability include, but are not limited to, anticancer drugs (everolimus); anti-infective agents halofantrine, maraviroc); statins (atorvastatin, lovastatin, and simvastatin); cardioactive drugs (amiodarone, clopidogrel, dronedarone, eplenorone, ticagrelor); HIV protease inhibitors (saquinavir), immunosuppressants (cyclosporine, sirolimus, tacrolimus, everolimus); antihistamines (terfenadine); gastrointestinal agents (domperidone); central nervous system agents (buspirone, dextromethorphan, oral ketamine, lurasidone, quetiapine, selective serotonin reuptake inhibitors [sertraline]); and urinary tract agents (darifenacin) (reviewed in 171). Because of the potential for adverse drug interactions, some clinicians recommend that people taking medications with low bioavailability (i.e., undergoing extensive metabolism by CYP3A4) avoid consuming grapefruit and grapefruit juice altogether during the treatment period (171).
Flavonoids can bind nonheme iron, inhibiting its intestinal absorption (172, 173). Nonheme iron is the principal form of iron in plant foods, dairy products, and iron supplements. The consumption of one cup of tea or cocoa with a meal has been found to decrease the absorption of nonheme iron in that meal by about 70% (174, 175). Flavonoids can also inhibit intestinal heme iron absorption (176). Interestingly, ascorbic acid greatly enhances the absorption of iron (see the article on Iron) and is able to counteract the inhibitory effect of flavonoids on nonheme and heme iron absorption (173, 176, 177). To maximize iron absorption from a meal or iron supplements, flavonoid-rich food and beverages and flavonoid supplements should not be consumed at the same time.
Originally written in 2005 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in June 2008 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2015 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in February 2016 by:
Alan Crozier, Ph.D.
Professor, Department of Nutrition
University of California, Davis
Copyright 2005-2024 Linus Pauling Institute
1. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013; 2013:162750. (PubMed)
2. Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727-747. (PubMed)
3. Xiao J, Kai G, Yamamoto K, Chen X. Advance in dietary polyphenols as α-glucosidases inhibitors: a review on structure-activity relationship aspect. Crit Rev Food Sci Nutr. 2013;53(8):818-836. (PubMed)
4. Rothwell JA, Urpi-Sarda M, Boto-Ordonez M, et al. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol Nutr Food Res. 2016;60(1):203-211. (PubMed)
5. Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51(2):454-463. (PubMed)
6. Williamson G. Common features in the pathways of absorption and metabolism of flavonoids. In: Meskin MS, R. BW, Davies AJ, Lewis DS, Randolph RK, eds. Phytochemicals: Mechanisms of Action. Boca Raton: CRC Press; 2004:21-33.
7. Nemeth K, Plumb GW, Berrin JG, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42(1):29-42. (PubMed)
8. Gonzales GB, Smagghe G, Grootaert C, Zotti M, Raes K, Van Camp J. Flavonoid interactions during digestion, absorption, distribution and metabolism: a sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab Rev. 2015;47(2):175-190. (PubMed)
9. Monagas M, Urpi-Sarda M, Sanchez-Patan F, et al. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1(3):233-253. (PubMed)
10. Bordenave N, Hamaker BR, Ferruzzi MG. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014;5(1):18-34. (PubMed)
11. Zhang H, Yu D, Sun J, et al. Interaction of plant phenols with food macronutrients: characterisation and nutritional-physiological consequences. Nutr Res Rev. 2014;27(1):1-15. (PubMed)
12. Xiao J, Mao F, Yang F, Zhao Y, Zhang C, Yamamoto K. Interaction of dietary polyphenols with bovine milk proteins: molecular structure-affinity relationship and influencing bioactivity aspects. Mol Nutr Food Res. 2011;55(11):1637-1645. (PubMed)
13. Lorenz M, Jochmann N, von Krosigk A, et al. Addition of milk prevents vascular protective effects of tea. Eur Heart J. 2007;28(2):219-223. (PubMed)
14. Roowi S, Stalmach A, Mullen W, Lean ME, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem. 2010;58(2):1296-1304. (PubMed)
15. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577-3584. (PubMed)
16. Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol Nutr Food Res. 2007;51(7):765-781. (PubMed)
17. Marin L, Miguelez EM, Villar CJ, Lombo F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int. 2015;2015:905215. (PubMed)
18. Inoue-Choi M, Yuan JM, Yang CS, et al. Genetic Association Between the COMT Genotype and Urinary Levels of Tea Polyphenols and Their Metabolites among Daily Green Tea Drinkers. Int J Mol Epidemiol Genet. 2010;1(2):114-123. (PubMed)
19. Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res. 2003;63(21):7526-7529. (PubMed)
20. Jiang W, Hu M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012;2(21):7948-7963. (PubMed)
21. Xiao J, Kai G. A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure-affinity relationship. Crit Rev Food Sci Nutr. 2012;52(1):85-101. (PubMed)
22. Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S-242S. (PubMed)
23. Kroon PA, Clifford MN, Crozier A, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80(1):15-21. (PubMed)
24. Heijnen CG, Haenen GR, van Acker FA, van der Vijgh WJ, Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro. 2001;15(1):3-6. (PubMed)
25. Chun OK, Kim DO, Lee CY. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem. 2003;51(27):8067-8072. (PubMed)
26. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133(10):3275S-3284S. (PubMed)
27. Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838-849. (PubMed)
28. Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41(12):1727-1746. (PubMed)
29. Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36(11):1199-1208. (PubMed)
30. Cheng IF, Breen K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals. 2000;13(1):77-83. (PubMed)
31. Spencer JP, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem. 2003;278(37):34783-34793. (PubMed)
32. Spencer JP, Schroeter H, Crossthwaithe AJ, Kuhnle G, Williams RJ, Rice-Evans C. Contrasting influences of glucuronidation and O-methylation of epicatechin on hydrogen peroxide-induced cell death in neurons and fibroblasts. Free Radic Biol Med. 2001;31(9):1139-1146. (PubMed)
33. Hou Z, Lambert JD, Chin KV, Yang CS. Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention. Mutat Res. 2004;555(1-2):3-19. (PubMed)
34. Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133(10):3262S-3267S. (PubMed)
35. Espley RV, Butts CA, Laing WA, et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144(2):146-154. (PubMed)
36. Kim MC, Kim SJ, Kim DS, et al. Vanillic acid inhibits inflammatory mediators by suppressing NF-kappaB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol Immunotoxicol. 2011;33(3):525-532. (PubMed)
37. Lee SG, Kim B, Yang Y, et al. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-kappaB independent of NRF2-mediated mechanism. J Nutr Biochem. 2014;25(4):404-411. (PubMed)
38. Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr Metab Cardiovasc Dis. 2012;22(1):72-80. (PubMed)
39. Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010;58(24):12722-12728. (PubMed)
40. Edirisinghe I, Banaszewski K, Cappozzo J, McCarthy D, Burton-Freeman BM. Effect of black currant anthocyanins on the activation of endothelial nitric oxide synthase (eNOS) in vitro in human endothelial cells. J Agric Food Chem. 2011;59(16):8616-8624. (PubMed)
41. Hidalgo M, Martin-Santamaria S, Recio I, et al. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012;7(2):295-306. (PubMed)
42. Chen XQ, Wang XB, Guan RF, et al. Blood anticoagulation and antiplatelet activity of green tea (-)-epigallocatechin (EGC) in mice. Food Funct. 2013;4(10):1521-1525. (PubMed)
43. Ahmad A, Khan RM, Alkharfy KM. Effects of selected bioactive natural products on the vascular endothelium. J Cardiovasc Pharmacol. 2013;62(2):111-121. (PubMed)
44. Hanhineva K, Torronen R, Bondia-Pons I, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11(4):1365-1402. (PubMed)
45. Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777-1789. (PubMed)
46. Delgado ME, Haza AI, Arranz N, Garcia A, Morales P. Dietary polyphenols protect against N-nitrosamines and benzo(a)pyrene-induced DNA damage (strand breaks and oxidized purines/pyrimidines) in HepG2 human hepatoma cells. Eur J Nutr. 2008;47(8):479-490. (PubMed)
47. Erba D, Casiraghi MC, Martinez-Conesa C, Goi G, Massaccesi L. Isoflavone supplementation reduces DNA oxidative damage and increases O-β-N-acetyl-D-glucosaminidase activity in healthy women. Nutr Res. 2012;32(4):233-240. (PubMed)
48. Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20(2):187-210. (PubMed)
49. Schwarz D, Kisselev P, Roots I. CYP1A1 genotype-selective inhibition of benzo[a]pyrene activation by quercetin. Eur J Cancer. 2005;41(1):151-158. (PubMed)
50. Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30(2):300-307. (PubMed)
51. Ravishankar D, Watson KA, Boateng SY, Green RJ, Greco F, Osborn HM. Exploring quercetin and luteolin derivatives as antiangiogenic agents. Eur J Med Chem. 2015;97:259-274. (PubMed)
52. Santos BL, Oliveira MN, Coelho PC, et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem Biol Interact. 2015;242:123-138. (PubMed)
53. Sokolov AN, Pavlova MA, Klosterhalfen S, Enck P. Chocolate and the brain: neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2445-2453. (PubMed)
54. Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3-4):115-126. (PubMed)
55. Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111(1):1-11. (PubMed)
56. Wang ZM, Zhao D, Nie ZL, et al. Flavonol intake and stroke risk: a meta-analysis of cohort studies. Nutrition. 2014;30(5):518-523. (PubMed)
57. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114(9):1496-1503. (PubMed)
58. US Department of Agriculture. USDA Database for the Proanthocyanidin Content of Selected Foods. August, 2004. Available at: http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/PA/PA.pdf. Accessed 8/25/15.
59. US Department of Agriculture. USDA Database for the Isoflavone Content of Selected Foods, release 2.0. September 2008. Available at: http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/isoflav/Isoflav_R2.pdf. Accessed 8/25/15.
60. US Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods, release 3.1. May 2014. Available at: http://www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/Flav/Flav_R03-1.pdf. Accessed 8/25/15.
61. Vogiatzoglou A, Mulligan AA, Bhaniani A, et al. Associations between flavan-3-ol intake and CVD risk in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk). Free Radic Biol Med. 2015;84:1-10. (PubMed)
62. Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr. 2015;102(1):172-181. (PubMed)
63. Grassi D, Desideri G, Ferri C. Protective effects of dark chocolate on endothelial function and diabetes. Curr Opin Clin Nutr Metab Care. 2013;16(6):662-668. (PubMed)
64. Sansone R, Rodriguez-Mateos A, Heuel J, et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study. Br J Nutr. 2015;114(8):1246-1255. (PubMed)
65. Basu A, Fu DX, Wilkinson M, et al. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res. 2010;30(7):462-469. (PubMed)
66. Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136(4):981-986. (PubMed)
67. Moazen S, Amani R, Homayouni Rad A, Shahbazian H, Ahmadi K, Taha Jalali M. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: a randomized double-blind controlled trial. Ann Nutr Metab. 2013;63(3):256-264. (PubMed)
68. Edirisinghe I, Banaszewski K, Cappozzo J, et al. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br J Nutr. 2011;106(6):913-922. (PubMed)
69. Karlsen A, Retterstol L, Laake P, et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137(8):1951-1954. (PubMed)
70. Basu A, Lyons TJ. Strawberries, blueberries, and cranberries in the metabolic syndrome: clinical perspectives. J Agric Food Chem. 2012;60(23):5687-5692. (PubMed)
71. Zhu Y, Ling W, Guo H, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013;23(9):843-849. (PubMed)
72. Qin Y, Xia M, Ma J, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90(3):485-492. (PubMed)
73. Zhu Y, Huang X, Zhang Y, et al. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J Clin Endocrinol Metab. 2014;99(2):561-569. (PubMed)
74. Curtis PJ, Kroon PA, Hollands WJ, et al. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. 2009;139(12):2266-2271. (PubMed)
75. Grassi D, Desideri G, Di Giosia P, et al. Tea, flavonoids, and cardiovascular health: endothelial protection. Am J Clin Nutr. 2013;98(6 Suppl):1660S-1666S. (PubMed)
76. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829-837, 837a-837d. (PubMed)
77. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344-351. (PubMed)
78. Liu Y, Li D, Zhang Y, Sun R, Xia M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am J Physiol Endocrinol Metab. 2014;306(8):E975-988. (PubMed)
79. Zhu Y, Xia M, Yang Y, et al. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011;57(11):1524-1533. (PubMed)
80. Ras RT, Zock PL, Draijer R. Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS One. 2011;6(3):e16974. (PubMed)
81. Hooper L, Kay C, Abdelhamid A, et al. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95(3):740-751. (PubMed)
82. Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46(2):398-405. (PubMed)
83. Grassi D, Desideri G, Necozione S, et al. Protective effects of flavanol-rich dark chocolate on endothelial function and wave reflection during acute hyperglycemia. Hypertension. 2012;60(3):827-832. (PubMed)
84. Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond). 2008;32(8):1289-1296. (PubMed)
85. West SG, McIntyre MD, Piotrowski MJ, et al. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr. 2014;111(4):653-661. (PubMed)
86. Flammer AJ, Sudano I, Wolfrum M, et al. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. 2012;33(17):2172-2180. (PubMed)
87. Schroeter H, Heiss C, Balzer J, et al. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103(4):1024-1029. (PubMed)
88. Gomez-Guzman M, Jimenez R, Sanchez M, et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic Biol Med. 2012;52(1):70-79. (PubMed)
89. Bachmair EM, Ostertag LM, Zhang X, de Roos B. Dietary manipulation of platelet function. Pharmacol Ther. 2014;144(2):97-113. (PubMed)
90. Pearson DA, Paglieroni TG, Rein D, et al. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106(4-5):191-197. (PubMed)
91. Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893. (PubMed)
92. Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. 2014;53(6):1299-1311. (PubMed)
93. Guerrero L, Castillo J, Quinones M, et al. Inhibition of angiotensin-converting enzyme activity by flavonoids: structure-activity relationship studies. PLoS One. 2012;7(11):e49493. (PubMed)
94. Egert S, Bosy-Westphal A, Seiberl J, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009;102(7):1065-1074. (PubMed)
95. Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137(11):2405-2411. (PubMed)
96. Zahedi M, Ghiasvand R, Feizi A, Asgari G, Darvish L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int J Prev Med. 2013;4(7):777-785. (PubMed)
97. Brull V, Burak C, Stoffel-Wagner B, et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br J Nutr. 2015;114(8):1263-1277. (PubMed)
98. Zamora-Ros R, Forouhi NG, Sharp SJ, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care. 2013;36(12):3961-3970. (PubMed)
99. Zamora-Ros R, Forouhi NG, Sharp SJ, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr. 2014;144(3):335-343. (PubMed)
100. Wang X, Tian J, Jiang J, et al. Effects of green tea or green tea extract on insulin sensitivity and glycaemic control in populations at risk of type 2 diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. J Hum Nutr Diet. 2014;27(5):501-512. (PubMed)
101. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98(2):340-348. (PubMed)
102. Zheng XX, Xu YL, Li SH, Hui R, Wu YJ, Huang XH. Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97(4):750-762. (PubMed)
103. Grassi D, Desideri G, Necozione S, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138(9):1671-1676. (PubMed)
104. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35(2):226-232. (PubMed)
105. Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr. 2012;95(4):925-933. (PubMed)
106. Hokayem M, Blond E, Vidal H, et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2013;36(6):1454-1461. (PubMed)
107. Soltani R, Gorji A, Asgary S, Sarrafzadegan N, Siavash M. Evaluation of the Effects of Cornus mas L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid Based Complement Alternat Med. 2015;2015:740954. (PubMed)
108. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145(4):742-748. (PubMed)
109. Yang CS, Yang GY, Landau JM, Kim S, Liao J. Tea and tea polyphenols inhibit cell hyperproliferation, lung tumorigenesis, and tumor progression. Exp Lung Res. 1998;24(4):629-639. (PubMed)
110. Balasubramanian S, Govindasamy S. Inhibitory effect of dietary flavonol quercetin on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Carcinogenesis. 1996;17(4):877-879. (PubMed)
111. Li ZG, Shimada Y, Sato F, et al. Inhibitory effects of epigallocatechin-3-gallate on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in F344 rats. Int J Oncol. 2002;21(6):1275-1283. (PubMed)
112. Yamane T, Nakatani H, Kikuoka N, et al. Inhibitory effects and toxicity of green tea polyphenols for gastrointestinal carcinogenesis. Cancer. 1996;77(8 Suppl):1662-1667. (PubMed)
113. Guo JY, Li X, Browning JD, Jr., et al. Dietary soy isoflavones and estrone protect ovariectomized ERαKO and wild-type mice from carcinogen-induced colon cancer. J Nutr. 2004;134(1):179-182. (PubMed)
114. Huang MT, Xie JG, Wang ZY, et al. Effects of tea, decaffeinated tea, and caffeine on UVB light-induced complete carcinogenesis in SKH-1 mice: demonstration of caffeine as a biologically important constituent of tea. Cancer Res. 1997;57(13):2623-2629. (PubMed)
115. Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98(18):10350-10355. (PubMed)
116. Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9(1):68-76. (PubMed)
117. Yamagishi M, Natsume M, Osakabe N, et al. Effects of cacao liquor proanthocyanidins on PhIP-induced mutagenesis in vitro, and in vivo mammary and pancreatic tumorigenesis in female Sprague-Dawley rats. Cancer Lett. 2002;185(2):123-130. (PubMed)
118. Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr. 2012;31(3):206-238. (PubMed)
119. Woo HD, Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J Gastroenterol. 2013;19(7):1011-1019. (PubMed)
120. Nimptsch K, Zhang X, Cassidy A, et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am J Clin Nutr. 2016;103(1):184-191. (PubMed)
121. Woo HD, Kim J. Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis. PLoS One. 2013;8(9):e75604. (PubMed)
122. Tang NP, Zhou B, Wang B, Yu RB, Ma J. Flavonoids intake and risk of lung cancer: a meta-analysis. Jpn J Clin Oncol. 2009;39(6):352-359. (PubMed)
123. Ollberding NJ, Lim U, Wilkens LR, et al. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst. 2012;104(1):67-76. (PubMed)
124. Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. (PubMed)
125. Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr. 2014;100(5):1344-1351. (PubMed)
126. Gates MA, Vitonis AF, Tworoger SS, et al. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2009;124(8):1918-1925. (PubMed)
127. Rossi M, Negri E, Lagiou P, et al. Flavonoids and ovarian cancer risk: A case-control study in Italy. Int J Cancer. 2008;123(4):895-898. (PubMed)
128. Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev. 2014;15(17):7001-7010. (PubMed)
129. Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2011;125(2):315-323. (PubMed)
130. Iwasaki M, Hamada GS, Nishimoto IN, et al. Isoflavone, polymorphisms in estrogen receptor genes and breast cancer risk in case-control studies in Japanese, Japanese Brazilians and non-Japanese Brazilians. Cancer Sci. 2009;100(5):927-933. (PubMed)
131. Wang Q, Li H, Tao P, et al. Soy isoflavones, CYP1A1, CYP1B1, and COMT polymorphisms, and breast cancer: a case-control study in southwestern China. DNA Cell Biol. 2011;30(8):585-595. (PubMed)
132. Hui C, Qi X, Qianyong Z, Xiaoli P, Jundong Z, Mantian M. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS One. 2013;8(1):e54318. (PubMed)
133. Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598-606. (PubMed)
134. Miyanaga N, Akaza H, Hinotsu S, et al. Prostate cancer chemoprevention study: an investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012;103(1):125-130. (PubMed)
135. Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545(1):51-64. (PubMed)
136. Nurk E, Refsum H, Drevon CA, et al. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009;139(1):120-127. (PubMed)
137. Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16(4):357-363. (PubMed)
138. Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(12):1364-1371. (PubMed)
139. Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60(3):794-801. (PubMed)
140. Mastroiacovo D, Kwik-Uribe C, Grassi D, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. Am J Clin Nutr. 2015;101(3):538-548. (PubMed)
141. Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat. 2008;4(2):433-440. (PubMed)
142. Crews WD, Jr., Harrison DW, Wright JW. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: clinical findings from a sample of healthy, cognitively intact older adults. Am J Clin Nutr. 2008;87(4):872-880. (PubMed)
143. Massee LA, Ried K, Pase M, et al. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: a randomized, controlled trial. Front Pharmacol. 2015;6:93. (PubMed)
144. Pase MP, Scholey AB, Pipingas A, et al. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharmacol. 2013;27(5):451-458. (PubMed)
145. Scholey AB, French SJ, Morris PJ, Kennedy DO, Milne AL, Haskell CF. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J Psychopharmacol. 2010;24(10):1505-1514. (PubMed)
146. Kent K, Charlton K, Roodenrys S, et al. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur J Nutr. 2015; Oct 19. [Epub ahead of print]. (PubMed)
147. Alharbi MH, Lamport DJ, Dodd GF, et al. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur J Nutr. 2015; Aug 18. [Epub ahead of print]. (PubMed)
148. Kean RJ, Lamport DJ, Dodd GF, et al. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am J Clin Nutr. 2015;101(3):506-514. (PubMed)
149. Casini ML, Marelli G, Papaleo E, Ferrari A, D'Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85(4):972-978. (PubMed)
150. Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, Bressel MA. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause. 2003;10(3):196-202. (PubMed)
151. Kim K, Vance TM, Chun OK. Estimated intake and major food sources of flavonoids among US adults: changes between 1999-2002 and 2007-2010 in NHANES. Eur J Nutr. 2015; May 31. [Epub ahead of print]. (PubMed)
152. Sebastian RS, Wilkinson Enns C, Goldman JD, et al. A New Database Facilitates Characterization of Flavonoid Intake, Sources, and Positive Associations with Diet Quality among US Adults. J Nutr. 2015;145(6):1239-1248. (PubMed)
153. Hendler SS, Rorvik DR, eds. PDR for Nutritional Supplements. 2nd ed: Thomson Reuters; 2008.
154. Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45(11):2179-2205. (PubMed)
155. Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54(6):960-963. (PubMed)
156. Ferry DR, Smith A, Malkhandi J, et al. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2(4):659-668. (PubMed)
157. Ottaviani JI, Balz M, Kimball J, et al. Safety and efficacy of cocoa flavanol intake in healthy adults: a randomized, controlled, double-masked trial. Am J Clin Nutr. 2015;102(6):1425-1435. (PubMed)
158. Jatoi A, Ellison N, Burch PA, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97(6):1442-1446. (PubMed)
159. Pisters KM, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19(6):1830-1838. (PubMed)
160. Sarma DN, Barrett ML, Chavez ML, et al. Safety of green tea extracts : a systematic review by the US Pharmacopeia. Drug Saf. 2008;31(6):469-484. (PubMed)
161. Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9(9):3312-3319. (PubMed)
162. Dostal AM, Samavat H, Bedell S, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol. 2015;83:26-35. (PubMed)
163. Li Y, Paxton JW. The effects of flavonoids on the ABC transporters: consequences for the pharmacokinetics of substrate drugs. Expert Opin Drug Metab Toxicol. 2013;9(3):267-285. (PubMed)
164. Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4(5):281-297. (PubMed)
165. Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75(1):13-33. (PubMed)
166. Freedman JE, Parker C, 3rd, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103(23):2792-2798. (PubMed)
167. Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J Nutr. 2000;130(1):53-56. (PubMed)
168. Polagruto JA, Schramm DD, Wang-Polagruto JF, Lee L, Keen CL. Effects of flavonoid-rich beverages on prostacyclin synthesis in humans and human aortic endothelial cells: association with ex vivo platelet function. J Med Food. 2003;6(4):301-308. (PubMed)
169. Murphy KJ, Chronopoulos AK, Singh I, et al. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77(6):1466-1473. (PubMed)
170. Natural Medicines. Hesperidin Professional Monograph; 2015.
171. Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185(4):309-316. (PubMed)
172. Kim EY, Ham SK, Shigenaga MK, Han O. Bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J Nutr. 2008;138(9):1647-1651. (PubMed)
173. Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr. 2008;87(4):881-886. (PubMed)
174. Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr. 1999;81(4):289-295. (PubMed)
175. Zijp IM, Korver O, Tijburg LB. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40(5):371-398. (PubMed)
176. Ma Q, Kim EY, Lindsay EA, Han O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J Food Sci. 2011;76(5):H143-150. (PubMed)
177. Kim EY, Ham SK, Bradke D, Ma Q, Han O. Ascorbic acid offsets the inhibitory effect of bioactive dietary polyphenolic compounds on transepithelial iron transport in Caco-2 intestinal cells. J Nutr. 2011;141(5):828-834. (PubMed)