Contents
See also: Immunity In Brief
The immune system protects the body against infection and disease. It is a complex and integrated system of cells, tissues, and organs that has specialized roles in defending against foreign substances and pathogenic microorganisms, including bacteria, viruses, and fungi. The immune system also functions to guard against the development of cancer. For these actions, the immune system must recognize foreign invaders, as well as abnormal cells and distinguish them from self (1). However, the immune system is a double-edged sword in that host tissues can be damaged in the process of combating and destroying invading pathogens. A key component of the immediate immune response is inflammation, which can cause damage to host tissues, although the damage is usually not significant (2). Inflammation is discussed in a separate article; this article focuses on nutrition and immunity.
Cells of the immune system originate in the bone marrow and circulate to peripheral tissues through the blood and lymph. Organs of the immune system include the thymus, spleen, and lymph nodes (3). T-lymphocytes develop in the thymus, which is located in the chest directly above the heart. The spleen, which is located in the upper abdomen, makes antibodies and removes old and damaged red blood cells (4). The immune system is broadly divided into two major components: innate immunity and adaptive immunity. Innate immunity involves immediate, nonspecific responses to foreign invaders, while adaptive immunity requires more time to develop its complex, specific responses (1).
Innate immunity is the first line of defense against foreign substances and pathogenic microorganisms. It is an immediate, nonspecific defense that does not involve immunologic memory of pathogens. Because of the lack of specificity, the actions of the innate immune system can result in damage to the body’s tissues (5). A lack of immunologic memory means that the same response is mounted regardless of how often a specific antigen is encountered (6).
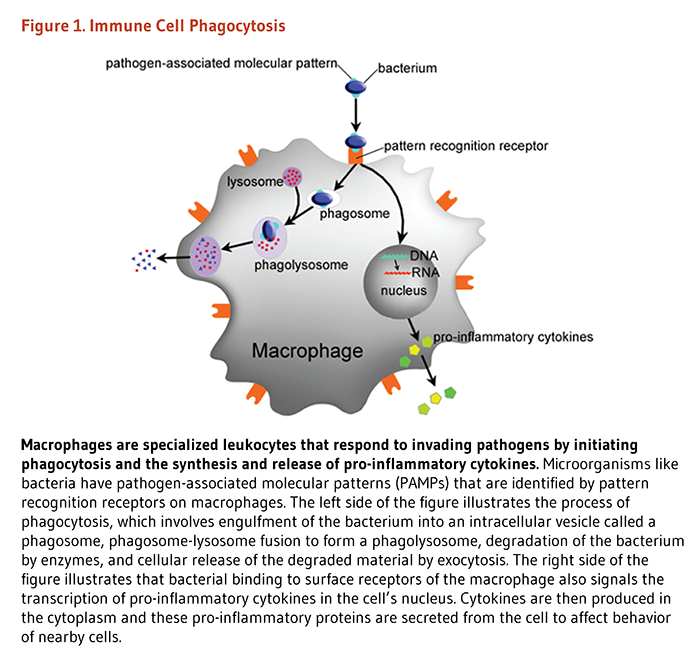
The innate immune system is comprised of various anatomical barriers to infection, including physical barriers (e.g., the skin), chemical barriers (e.g., acidity of stomach secretions), and biological barriers (e.g., normal microflora of the gastrointestinal tract) (1). In addition to anatomical barriers, the innate immune system is comprised of soluble factors and phagocytic cells that form the first line of defense against pathogens. Soluble factors include the complement system, acute-phase proteins, and messenger proteins called cytokines (6). The complement system, a biochemical network of more than 30 proteins in plasma and on cellular surfaces, is a key component of innate immunity. The complement system elicits responses that kill invading pathogens by direct lysis (cell rupture) or by promoting phagocytosis. Complement proteins also regulate inflammatory responses, which are part of innate immunity (7-9). Acute-phase proteins are a class of plasma proteins that are important in inflammation. Cytokines secreted by immune cells in the early stages of inflammation stimulate the synthesis of acute-phase proteins in the liver (10). Cytokines are chemical messengers that have key roles in regulating the immune response; some cytokines directly fight pathogens. For example, some interferons have antiviral activity (6). These soluble factors are important in recruiting phagocytic cells to local areas of infection. Monocytes, macrophages, and neutrophils are key immune cells that engulf and digest invading microorganisms in the process called phagocytosis. These cells express surface receptors that identify pattern recognition receptors that are unique to pathogenic microorganisms but conserved across several families of pathogens (Figure 1) (2, 11). For more information about the innate immune response, see the article on Inflammation.
Adaptive immunity (also called acquired immunity), a second line of defense against pathogens, takes several days or weeks to fully develop. However, adaptive immunity is much more complex than innate immunity because it involves antigen-specific responses and immunologic "memory." Exposure to a specific antigen on an invading pathogen stimulates production of immune cells that target the pathogen for destruction (1). Immunologic “memory” means that immune responses upon a second exposure to the same pathogen are faster and stronger because antigens are "remembered." Primary mediators of the adaptive immune response are B lymphocytes (B cells) and T lymphocytes (T cells). B cells produce antibodies, which are specialized proteins that recognize and bind to foreign proteins or pathogens in order to neutralize them or mark them for destruction by macrophages. The response mediated by antibodies is called humoral immunity. In contrast, cell-mediated immunity is carried out by T cells — lymphocytes that develop in the thymus. Different subgroups of T cells have different roles in adaptive immunity. For instance, cytotoxic T cells (killer T cells) directly attack and kill infected cells, while helper T cells enhance the responses and thus aid in the function of other lymphocytes (5, 6). Regulatory T cells, sometimes called suppressor T cells, suppress immune responses (4). In addition to its vital role in innate immunity, the complement system modulates adaptive immune responses and is one example of the interplay between the innate and adaptive immune systems (7, 12). Components of both innate and adaptive immunity interact and work together to protect the body from infection and disease.
Nutritional status can modulate the actions of the immune system; therefore, the sciences of nutrition and immunology are tightly linked (reviewed in 13). In fact, malnutrition is the most common cause of immunodeficiency in the world (14), and chronic malnutrition is a major risk factor for global morbidity and mortality (15). More than 800 million people are estimated to be undernourished, most in the developing world (16), but undernutrition is also a problem in industrialized nations, especially in hospitalized individuals and the elderly (17). Poor overall nutrition can lead to inadequate intake of energy and macronutrients, as well as deficiencies in certain micronutrients that are required for proper immune function. Such nutrient deficiencies can result in immunosuppression and dysregulation of immune responses. In particular, deficiencies in certain nutrients can impair phagocytic function in innate immunity and adversely affect several aspects of adaptive immunity, including cytokine production, as well as antibody- and cell-mediated immunities (18, 19). Overnutrition, a form of malnutrition where nutrients, specifically macronutrients, are provided in excess of dietary requirements, also negatively impacts immune system functions (see Overnutrition and obesity).
Impaired immune responses induced by malnutrition can increase one’s susceptibility to infection and illness. Infection and illness can, in turn, exacerbate states of malnutrition, for example, by reducing nutrient intake through diminished appetite, impairing nutrient absorption, increasing nutrient losses, or altering the body’s metabolism such that nutrient requirements are increased (19). Thus, states of malnutrition and infection can aggravate each other and lead to a vicious cycle (14).
Protein-energy malnutrition (PEM; also sometimes called protein-calorie malnutrition) is a common nutritional problem that principally affects young children and the elderly (20, 21). According to the World Health Organization, more than one-third of the world’s underprivileged, preschool age children (80% living in Asia, 15% in Africa, and 5% in Latin America) are affected by PEM. This malnutrition stunts physical growth and mental development, with lasting effects into adulthood (22). Clinical conditions of severe PEM are termed marasmus, kwashiorkor, or a hybrid of these two syndromes. Marasmus is a wasting disorder that is characterized by depletion of fat stores and muscle wasting. It results from a deficiency in both protein and calories (i.e., all nutrients). Individuals afflicted with marasmus appear emaciated and are grossly underweight and do not present with edema (23). In contrast, a hallmark of kwashiorkor is the presence of edema. Kwashiorkor is primarily caused by a deficiency in dietary protein, while overall caloric intake may be normal (23, 24). Both forms are more common in developing nations, but certain types of PEM are also present in various subgroups in industrialized nations, such as the elderly and individuals who are hospitalized (17). In the developed world, PEM more commonly occurs secondary to a chronic disease that interferes with nutrient metabolism, such as inflammatory bowel disease, chronic renal failure, or cancer (24). Thus, when treating PEM in connection with chronic disease, it is necessary to address the underlying cause of malnutrition, as well as correct the associated nutrient deficits (25, 26).
Regardless of the specific cause, PEM significantly increases susceptibility to infection by adversely affecting aspects of both innate immunity and adaptive immunity (15). With respect to innate immunity, PEM has been associated with reduced production of certain cytokines and several complement proteins, as well as impaired phagocyte function (20, 27, 28). Such malnutrition disorders can also compromise the integrity of mucosal barriers, increasing vulnerability to infections of the respiratory, gastrointestinal, and urinary tracts (23). With respect to adaptive immunity, PEM primarily affects cell-mediated aspects instead of components of humoral immunity. In particular, PEM leads to atrophy of the thymus, the organ that produces T cells, which reduces the number of circulating T cells and decreases the effectiveness of the memory response to antigens (23, 28). PEM also compromises functions of other lymphoid tissues, including the spleen and lymph nodes (20). While humoral immunity is affected to a lesser extent, antibody affinity and response is generally decreased in PEM (28). It is important to note that PEM usually occurs in combination with deficiencies in essential micronutrients, especially vitamin A, vitamin B6, folate, vitamin E, zinc, iron, copper, and selenium (23).
Experimental studies have shown that several types of dietary lipids (fatty acids) can modulate the immune response (29). Fatty acids that have this role include the long-chain polyunsaturated fatty acids (PUFAs) of the omega-3 and omega-6 classes. PUFAs are fatty acids with more than one double bond between carbons. In all omega-3 fatty acids, the first double bond is located between the third and fourth carbon atom counting from the methyl end of the fatty acid (n-3). Similarly, the first double bond in all omega-6 fatty acids is located between the sixth and seventh carbon atom from the methyl end of the fatty acid (n-6) (30). Humans lack the ability to place a double bond at the n-3 or n-6 positions of a fatty acid; therefore, fatty acids of both classes are considered essential nutrients and must be derived from the diet (For more information, see the article on Essential Fatty Acids) (30).
α-Linolenic acid (ALA) is a nutritionally essential n-3 fatty acid, and linoleic acid (LA) is a nutritionally essential n-6 fatty acid; dietary intake recommendations for essential fatty acids are for ALA and LA. Other fatty acids in the n-3 and n-6 classes can be endogenously synthesized from ALA or LA (see Figure 3 in the article on essential fatty acids). For instance the long-chain n-6 PUFA, arachidonic acid, can be synthesized from LA, and the long-chain n-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can be synthesized from ALA (30). However, synthesis of EPA and, especially, DHA may be insufficient under certain conditions, such as during pregnancy and lactation (31, 32). EPA and DHA, like other PUFAs, modulate cellular function, including immune and inflammatory responses (33).
Long-chain PUFAs are incorporated into membrane phospholipids of immune cells, where they modulate cell signaling of immune and inflammatory responses, such as phagocytosis and T-cell signaling. They also modulate the production of eicosanoids and other lipid mediators (33-35). Eicosanoids are 20-carbon PUFA derivatives that play key roles in inflammatory and immune responses. During an inflammatory response, long-chain PUFAs (e.g., arachidonic acid [AA] of the n-6 series and EPA of the n-3 series) in immune cell membranes can be metabolized by enzymes to form eicosanoids (e.g., prostaglandins, leukotrienes, and thromboxanes), which have varying effects on inflammation (33). Eicosanoids derived from AA can also regulate B- and T-cell functions. Resolvins are lipid mediators derived from EPA and DHA that appear to have anti-inflammatory properties (34). To a certain extent, the relative production of these lipid mediators can be altered by dietary and supplemental intake of lipids. In those who consume a typical Western diet, the amount of AA in immune cell membranes is much greater than the amount of EPA, which results in the formation of more eicosanoids derived from AA than EPA. However, increasing n-3 fatty acid intake dose-dependently increases the EPA content of immune cell membranes. The resulting effect would be increased production of eicosanoids derived from EPA and decreased production of eicosanoids derived from AA, leading to an overall anti-inflammatory effect (34, 36). While eicosanoids derived from EPA are less biologically active than AA-derived eicosanoids (37), supplementation with EPA and other n-3 PUFAs may nevertheless have utility in treating various inflammatory diseases. This is currently an active area of investigation; see the article on Essential Fatty Acids.
While n-3 PUFA supplementation may benefit individuals with inflammatory or autoimmune diseases, high n-3 PUFA intakes could possibly impair host-defense mechanisms and increase vulnerability to infectious disease (29, 38). Animal studies demonstrate that high dietary intake of EPA and DHA results in decreased pathogen clearance and increased susceptibility to experimental infections (39). In humans, a small number of randomized controlled trials (RCTs) indicate that EPA in particular may suppress some aspects of the immune response. Moreover, dose and participant age appear to be important modulating factors. In a randomized, double-blind, placebo-controlled trial in 46 healthy older men and women (ages 55 to 75 years), a moderate amount of EPA+DHA (720 mg EPA + 280 mg DHA/day in fish oil for 12 weeks) reduced NK cell activity (40) and T lymphocyte proliferation (41) but had no effect on inflammatory cell (neutrophil, monocyte, and macrophage) numbers, respiratory burst, or cytokine (TNF-α, IL-1β, and IL-6) production (42); DHA supplementation alone had no effect on any of the above-mentioned parameters. On the other hand, an RCT in young men (N=93, ages 18 to 42 years) found that 12 weeks of daily supplementation with up to 4 g of EPA and 0.9 g of DHA had no effect on NK cell activity, T lymphocyte proliferation, or cytokine production (43). Directly comparing healthy young (N=93, ages 18 to 42 years) and older (N=62, ages 53 to 70 years) men, another RCT found that supplementation with increasing doses of EPA-rich oil (that also contained some DHA) for 12 weeks had differential effects on immune parameters (44). EPA was more readily incorporated into mononuclear cell membranes in older men, and a reduced neutrophil respiratory burst (approximate 20% impairment) was observed at the two higher doses of supplementation (2.7 g/day and 4.05 g/day of EPA) in older men only (44). Supplementation with 1.35 g/day of EPA + 0.3 g/day of DHA did not affect innate immune functions in either young or older men.
In addition to PUFAs, isomers of LA called conjugated linoleic acid (CLA) have been shown to modulate immune function, mainly in animal and in vitro studies (45). CLA is found naturally in meat and milk of ruminant animals, but it is also available as a dietary supplement that contains two isomers, cis-9,trans-11 CLA and trans-10,cis-12 CLA. One study in 28 men and women found that CLA supplementation (3 g/day of a 50:50 mixture of the two main CLA isomers) was associated with an increase in plasma concentrations of IgA and IgM (46), two classes of antibodies. CLA supplementation was also associated with a decrease in levels of two pro-inflammatory cytokines and an increase in levels of an anti-inflammatory cytokine (46). Similar effects on the immune response have been observed in some animal studies (47, 48); however, a few other human studies have not found beneficial effects of CLA on various measures of immune status and function (49-51). More research is needed to understand the effects of CLA on the human immune response.
Further, lipids in general have a number of other roles in immunity besides being the precursors of eicosanoids and similar immune mediators. For instance, lipids are metabolized by immune cells to generate energy and are also important structural and functional components of cell membranes. Moreover, lipids can regulate gene expression through stimulation of membrane receptors or through modification of transcription factor activity. Further, lipids can covalently modify proteins, thereby affecting their function (34).
Deficiencies in select micronutrients (vitamins and nutritionally essential minerals) can adversely affect aspects of both innate and adaptive immunity, increasing vulnerability to infection and disease. Micronutrient deficiencies are quite common in the general US population, but especially in the poor, the elderly, and those who are obese (see Overnutrition and obesity) (52, 53). According to data from the US National Health and Nutrition Examination Survey (NHANES), 93% of the US adult population does not meet the estimated average requirement (EAR) for vitamin E, 56% for magnesium, 44% for vitamin A, 31% for vitamin C, 14% for vitamin B6, and 12% for zinc (54). Moreover, vitamin D deficiency is a major problem in the US and elsewhere; it has been estimated that 1 billion people in the world have either vitamin D deficiency or insufficiency (55). Because micronutrients play crucial roles in the development and expression of immune responses, selected micronutrient deficiencies can cause immunosuppression and thus increased susceptibility to infection and disease. The roles of several micronutrients in immune function are addressed below.
Vitamin A and its metabolites play critical roles in both innate and adaptive immunity. In innate immunity, the skin and mucosal cells of the eye and respiratory, gastrointestinal, and genitourinary tracts function as a barrier against infections. Vitamin A helps to maintain the structural and functional integrity of these mucosal cells. Vitamin A is also important to the normal function of several types of immune cells important in the innate response, including natural killer (NK) cells, macrophages, and neutrophils. Moreover, vitamin A is needed for proper function of cells that mediate adaptive immunity, such as T and B lymphocytes; thus, vitamin A is necessary for the generation of antibody responses to specific antigens (56).
Most of the immune effects of vitamin A are carried out by vitamin A derivatives, namely isomers of retinoic acid. Isomers of retinoic acid are steroid hormones that bind to retinoid receptors that belong to two different classes: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). In the classical pathway, RAR must first heterodimerize with RXR and then bind to small sequences of DNA called retinoic acid response elements (RAREs) to initiate a cascade of molecular interactions that modulate the transcription of specific genes (57). More than 500 genes are directly or indirectly regulated by retinoic acid (58). Several of these genes control cellular proliferation and differentiation; thus, vitamin A has obvious importance in immunity.
Vitamin A deficiency is a major public health problem worldwide, especially in developing nations, where availability of foods containing preformed vitamin A is limited (for information on sources of vitamin A, see the article on Vitamin A). Experimental studies in animal models, along with epidemiological studies, have shown that vitamin A deficiency leads to immunodeficiency and increases the risk of infectious diseases (56). In fact, deficiency in this micronutrient is a leading cause of morbidity and mortality among infants, children, and women in developing nations. Vitamin A-deficient individuals are vulnerable to certain infections, such as measles, malaria, and diarrheal diseases (56). Subclinical vitamin A deficiency might increase risk of infection as well (59). Infections can, in turn, lead to vitamin A deficiency in a number of different ways, for example, by reducing food intake, impairing vitamin absorption, increasing vitamin excretion, interfering with vitamin utilization, or increasing metabolic requirements of vitamin A (60).
Many of the specific effects of vitamin A deficiency on the immune system have been elucidated using animal models. Vitamin A deficiency impairs components of innate immunity. As mentioned above, vitamin A is essential in maintaining the mucosal barriers of the innate immune system. Thus, vitamin A deficiency compromises the integrity of this first line of defense, thereby increasing susceptibility to some types of infection, such as eye, respiratory, gastrointestinal, and genitourinary infections (61-67). Vitamin A deficiency results in reductions in both the number and killing activity of NK cells, as well as the function of neutrophils and other cells that phagocytose pathogens like macrophages. Specific measures of functional activity affected appear to include chemotaxis, phagocytosis, and immune cell ability to generate oxidants that kill invading pathogens (56). In addition, cytokine signaling may be altered in vitamin A deficiency, which would affect inflammatory responses of innate immunity.
Additionally, vitamin A deficiency impairs various aspects of adaptive immunity, including humoral and cell-mediated immunity. In particular, vitamin A deficiency negatively affects the growth and differentiation of B cells, which are dependent on retinol and its metabolites (68, 69). Vitamin A deficiency also affects B cell function; for example, animal experiments have shown that vitamin A deficiency impairs antibody responses (70-72). With respect to cell-mediated immunity, retinol is important in the activation of T cells (73), and vitamin A deficiency may affect cell-mediated immunity by decreasing the number or distribution of T cells, altering cytokine production, or by decreasing the expression of cell-surface receptors that mediate T-cell signaling (56).
Vitamin A supplementation enhances immunity and has been shown to reduce the infection-related morbidity and mortality associated with vitamin A deficiency. A meta-analysis of 43 randomized controlled trials (RCTs) that included approximately 215,633 children 6 months to 5 years of age found that vitamin A supplementation decreased the risk of all-cause mortality by 24% (RR: 0.76, 95% CI: 0.69 to 0.83); this analysis also found that vitamin A supplementation reduced the incidence of diarrhea (RR: 0.85, 95% CI: 0.82 to 0.87) and measles (RR: 0.50, 95% CI: 0.30 to 0.67) (56, 74-76). Vitamin A supplementation of mothers (during pregnancy or lactation), and early infants (1-5 months) does not appear to significantly affect child mortality, while supplementation of neonates (0 to 28 days) may reduce childhood mortality at 6 months of age (77, 78). Vitamin A supplementation is not beneficial in those with lower respiratory infections, such as pneumonia (77), and supplementation may actually aggravate the condition (56, 79-81). Although the effectiveness of vitamin A supplementation in children older than 6 months of age in low- and middle-income countries is now well established, the most effective dose and frequency of supplemental vitamin A delivery is still a matter of debate (56, 74, 78-80). For more information on vitamin A supplementation and childhood morbidity and mortality, see the article on Vitamin A. Because of potential adverse effects, vitamin A supplements should be reserved for undernourished populations and those with evidence of vitamin A deficiency (76). For information on vitamin A toxicity, see the article on Vitamin A.
Like vitamin A, the active form of vitamin D, 1,25-dihydroxyvitamin D3, functions as a steroid hormone to regulate expression of target genes. Many of the biological effects of 1,25-dihydroxyvitamin D3 are mediated through a nuclear transcription factor known as the vitamin D receptor (VDR) (82). Upon entering the nucleus of a cell, 1,25-dihydroxyvitamin D3 associates with the VDR and promotes its association with the retinoid X receptor (RXR). In the presence of 1,25-dihydroxyvitamin D3, the VDR/RXR complex binds small sequences of DNA known as vitamin D response elements (VDREs) and initiates a cascade of molecular interactions that modulate the transcription of specific genes. Thousands of VDREs have been identified throughout the genome, and VDR activation by 1,25-dihydroxyvitamin D is thought to directly and/or indirectly regulate 100 to 1,250 genes (83).
In addition to its effects on mineral homeostasis and bone metabolism, 1,25-dihydroxyvitamin D3 is now recognized to be a potent modulator of the immune system. The VDR is expressed in several types of immune cells, including monocytes, macrophages, dendritic cells, and activated T cells (84-87). Macrophages also produce the 25-hydroxyvitamin D3-1-hydroxylase enzyme, allowing for local conversion of vitamin D to its active form (88). Studies have demonstrated that 1,25-dihydroxyvitamin D3 modulates both innate and adaptive immune responses.
Antimicrobial peptides (AMPs) and proteins are critical components of the innate immune system because they directly kill pathogens, especially bacteria, and thereby enhance immunity (89). AMPs also modulate immune functions through cell-signaling effects (90). The active form of vitamin D regulates two important antimicrobial proteins, cathelicidin and defensin (91-94). Vitamin D has also been shown to stimulate other components of innate immunity, including immune cell proliferation and cytokine production (95). Through these roles, vitamin D helps protect against infections caused by pathogens.
Vitamin D has mainly inhibitory effects on adaptive immunity. In particular, 1,25-dihydroxyvitamin D3 suppresses antibody production by B cells and also inhibits proliferation of T cells in vitro (96-98). Moreover, 1,25-dihydroxyvitamin D3 has been shown to modulate the functional phenotype of helper T cells and dendritic cells (90). T cells that express the cell-surface protein CD4 are divided into two subsets depending on the particular cytokines that they produce: T helper (Th)1 cells are primarily involved in activating macrophages and inflammatory responses, and Th2 cells are primarily involved in stimulating antibody production by B cells (4). Some studies have shown that 1,25-dihydroxyvitamin D3 inhibits the development and function of Th1 cells (99, 100) but enhances the development and function of Th2 cells (101, 102) and regulatory T cells (103, 104). Because these latter cell types are important regulators in autoimmune disease and graft rejections, vitamin D is suggested to have utility in preventing and treating such conditions (105). Studies employing various animal models of autoimmune diseases and transplantation have reported beneficial effects of 1,25-dihydroxyvitamin D3 (reviewed in 100).
Indeed, vitamin D deficiency has been implicated in the development of certain autoimmune diseases, such as insulin-dependent diabetes mellitus (IDDM; type 1 diabetes mellitus), multiple sclerosis (MS), systemic lupus erythematosis (SLE), and rheumatoid arthritis (RA). Autoimmune diseases occur when the body mounts an immune response against its own tissue instead of a foreign pathogen. The targets of the inappropriate immune response are the insulin-producing β-cells of the pancreas in IDDM, the myelin-producing cells of the central nervous system in MS, multiple tissues and organs in SLE, and the collagen-producing cells of the joints in RA (106, 107).
Several case-control studies have found low serum 25-hydroxyvitamin D3 concentrations in individuals with IDDM, MS, and SLE; among these individuals, lower serum 25-hydroxyvitamin D3 concentrations have been associated with increased disease activity and relapse rates (108-110). Both genetic factors (e.g., VDR polymorphisms) and environment (e.g., low UVB exposure, low dietary vitamin D intake) may contribute to the low vitamin D status observed in these autoimmune diseases (110). For example, some observational studies have found the prevalence of various autoimmune conditions increases as latitude increases (111, 112). This suggests that lower exposure to ultraviolet-B radiation (the type of radiation needed to induce vitamin D synthesis in skin) and the associated decrease in endogenous vitamin D synthesis may play a role in the pathology of autoimmune diseases.
Low vitamin D intake has also been associated with an increased risk of MS (113), IDDM (114), and RA (115-121). Despite the well-documented relationship between low vitamin D status and autoimmune disease, few randomized controlled trials (RCTs) have been conducted, and the clinical efficacy of vitamin D supplementation in autoimmune prevention and treatment remains uncertain (108). A 2013 meta-analysis of RCTs that tested the effect of high-dose vitamin D supplements on the risk of relapse in MS patients included only five RCTs (with a total of 129 patients and 125 controls) and found no significant effect (122). A 2013 systematic review similarly reported no effect of vitamin D supplementation on MS relapse (123). At this time, we await the results of three ongoing RCTs evaluating the efficacy of vitamin D supplementation on disease progression and activity in individuals with relapsing remitting MS (124-126). For more information on the specific autoimmune conditions discussed above, see the article on Vitamin D.
Vitamin C is a highly effective antioxidant that protects the body’s cells against reactive oxygen species that are generated by immune cells to kill pathogens. Primarily through this role, the vitamin affects several components of innate and adaptive immunity; for example, vitamin C has been shown to stimulate both the production (127-131) and function (132, 133) of leukocytes (white blood cells), especially neutrophils, lymphocytes, and phagocytes. Specific measures of functions stimulated by vitamin C include cellular motility (132), chemotaxis (132, 133), and phagocytosis (133). Neutrophils, which attack foreign bacteria and viruses, seem to be the primary cell type stimulated by vitamin C, but lymphocytes and other phagocytes are also affected (134-136). In support of vitamin C’s effect on neutrophil function, supplementation with vitamin C-rich SunGold Kiwifruit (2 kiwifruit/day, providing approximately 259 mg/day of vitamin C, for four weeks) increased plasma and neutrophil vitamin C concentration and improved neutrophil chemotaxis and oxidant generation in 14 young men considered to have suboptimal vitamin C status (<50 µmol/L) at baseline (137).
Additionally, several studies have shown that supplemental vitamin C increases serum levels of antibodies (138, 139) and C1q complement proteins (140-142) in guinea pigs, which — like humans — cannot synthesize vitamin C and hence depend on dietary vitamin C. However, some studies have reported no beneficial changes in leukocyte production or function with vitamin C treatment (143-146). Vitamin C may also protect the integrity of immune cells. Neutrophils, mononuclear phagocytes, and lymphocytes accumulate vitamin C to high concentrations, which can protect these cell types from oxidative damage (131, 147, 148). In response to invading microorganisms, phagocytic leukocytes release non-specific toxins, such as superoxide radicals, hypochlorous acid (“bleach”), and peroxynitrite; these reactive oxygen species kill pathogens and, in the process, can damage the leukocytes themselves (149). Vitamin C, through its antioxidant functions, has been shown to protect leukocytes from such effects of autooxidation (150). Phagocytic leukocytes also produce and release cytokines, including interferons that have antiviral activity (151). Vitamin C has been shown to increase interferon levels in vitro (152). Further, vitamin C regenerates the antioxidant vitamin E from its oxidized form (153).
It is widely thought by the general public that vitamin C boosts the function of the immune system, and accordingly, may protect against viral infections and perhaps other diseases. While some studies suggest the biological plausibility of vitamin C as an immune enhancer, human studies published to date are conflicting. A 2013 review identified six trials, only two of which were double-blind, placebo-controlled, randomized controlled trials, that could be used to evaluate the effect of vitamin C supplementation on the prevention or treatment of pneumonia (154). Overall, vitamin C supplementation had a mild beneficial effect compared to placebo. However, methodological shortcomings and the highly variable characteristics of the study populations prevent comparisons and firm conclusions from the available data. At this time, prophylactic use of vitamin C supplements for pneumonia prevention and treatment cannot be advocated for the general population. For a review of vitamin C and the common cold, see the article on Vitamin C.
Vitamin E is a lipid-soluble antioxidant that protects the integrity of cell membranes from damage caused by free radicals (155). In particular, the α-tocopherol form of vitamin E protects against peroxidation of polyunsaturated fatty acids, which can potentially cause cellular damage and subsequently lead to improper immune responses (156). Several studies in animal models, as well as humans, indicate that vitamin E deficiency impairs both humoral and cell-mediated aspects of adaptive immunity, including B and T cell function (reviewed in 156). Moreover, vitamin E supplementation in excess of current intake recommendations has been shown to enhance immunity and decrease susceptibility to certain infections, especially in elderly individuals.
The natural age-related decline of the immune function is accompanied by an increased susceptibility to infections, a poorer response to immunization, and higher risks of developing cancers and autoimmune diseases. α-Tocopherol has been shown to enhance specifically the T cell-mediated immune response that declines with advancing age (reviewed in 157). T cell impaired response has been partly associated with a reduced capacity of naïve T cells to be activated during antigen presentation, and to produce interleukin-2 (IL-2) and proliferate as a result (158). However, very few studies have addressed the potential association between α-tocopherol and immune function in humans (157). In a small intervention study in older adults (mean age, 70 years), supplementation with 200 mg/day of all-rac-α-tocopherol (equivalent to 100 mg of RRR-α-tocopherol) for three months significantly improved natural killer (NK) cytotoxic activity, neutrophil chemotaxis, phagocytic response, and enhanced mitogen-induced lymphocyte proliferation and interleukin-2 (IL-2) production compared to baseline (159). In an earlier trial, daily supplementation of healthy older adults (≥65 years of age) with 200 mg of all-rac-α-tocopherol for 235 days also improved T lymphocyte-mediated immunity — as measured with the delayed-type hypersensitivity (DTH) skin test — and increased the production of antibodies in response to hepatitis B and tetanus vaccines (160).
Lower α-tocopherol doses failed to improve the DTH response compared to a placebo in another study in healthy participants (ages, 65-80 years) (161). A randomized, placebo-controlled trial in 617 nursing home residents (≥65 years of age) reported that daily supplementation with 200 IU of synthetic α-tocopherol (90 mg of RRR-α-tocopherol) for one year significantly lowered the risk of contracting upper respiratory tract infections, especially the common cold, but had no effect on lower respiratory tract (lung) infections (157, 160, 162-168). Yet, other trials have not reported an overall beneficial effect of vitamin E supplements on respiratory tract infections in older adults (161, 169-171). More research is needed to examine whether supplemental vitamin E might enhance immune function and reduce risk of infection in older adults.
Vitamin B6 is required in the endogenous synthesis and metabolism of amino acids — the building blocks of proteins like cytokines and antibodies. Animal and human studies have demonstrated that vitamin B6 deficiency impairs aspects of adaptive immunity, including both humoral and cell-mediated immunity. Specifically, deficiency in this micronutrient has been shown to affect lymphocyte proliferation, differentiation, and maturation as well as cytokine and antibody production (172-174). Correcting the vitamin deficiency restores the affected immune functions (174).
Additionally, several enzymatic reactions in the tryptophan-kynurenine pathway are dependent on vitamin B6 coenzyme, pyridoxal 5’-phosphate (PLP). This pathway is known to be activated during pro-inflammatory immune responses and plays a critical role in immune tolerance of the fetus during pregnancy (175). Key intermediates in the tryptophan-kynurenine pathway are involved in the regulation of immune responses. Several tryptophan derivatives have been found to induce the death (apoptosis) or block the proliferation of certain types of immune cells, such as lymphocytes (in particular T-helper 1). They can also inhibit the production of pro-inflammatory cytokines (reviewed in 175). There is evidence to suggest that adequate vitamin B6 intake is important for optimal immune system function, especially in older individuals (176, 177). Furthermore, chronic inflammation that triggers tryptophan degradation and underlies many diseases (e.g., cardiovascular disease and cancer) may precipitate the loss of PLP and increase vitamin B6 requirements. Additional research is needed to evaluate whether vitamin B6 intakes higher than the current RDA could prevent and/or reverse immune system impairments.
The B vitamin, folate, is required as a coenzyme to mediate the transfer of one-carbon units. Folate coenzymes act as acceptors and donors of one-carbon units in a variety of reactions critical to the endogenous synthesis and metabolism of nucleic acids (DNA and RNA) and amino acids (178, 179). Thus, folate has obvious importance in immunity. Animal studies and a few observational studies in humans indicate that folate deficiency is associated with an increased susceptibility to infection (180). Clinical folate deficiency, known as megaloblastic anemia, results in impaired immune responses, primarily affecting cell-mediated immunity; correcting the vitamin deficiency with folic acid supplementation restores the affected immune functions (181). Animal studies indicate that antibody responses of humoral immunity may also be impaired in folate deficiency, though human studies in this regard are lacking (180).
In humans, vitamin B12 functions as a coenzyme for two enzymatic reactions. One of the vitamin B12-dependent enzymes is involved in the synthesis of the amino acid, methionine, from homocysteine. Methionine in turn is required for the synthesis of S-adenosylmethionine, a methyl group donor used in many biological methylation reactions, including the methylation of a number of sites within DNA and RNA. The other vitamin B12-dependent enzyme, L-methylmalonyl-CoA mutase, converts L-methylmalonyl-CoA to succinyl-CoA, a compound that is important in the production of energy from fats and proteins, as well as in the synthesis of hemoglobin — the oxygen carrying pigment in red blood cells (182). Patients with diagnosed vitamin B12 deficiency (as pernicious anemia or megaloblastic anemia) have been reported to have suppressed natural killer cell activity and decreased numbers of circulating lymphocytes (183, 184). One study found that these immunomodulatory effects were corrected by treating the vitamin deficiency (183).
Zinc is critical for normal development and function of cells that mediate both innate and adaptive immunity (185). The cellular functions of zinc can be divided into three categories: (1) catalytic, (2) structural, and (3) regulatory (see the article on Zinc) (186). Because zinc is not stored in the body, regular dietary intake of the mineral is important in maintaining the integrity of the immune system. Thus, inadequate intake can lead to zinc deficiency and compromised immune responses (187). With respect to innate immunity, zinc deficiency impairs the complement system, cytotoxicity of natural killer cells, phagocytic activity of neutrophils and macrophages, and immune cell ability to generate oxidants that kill invading pathogens (188-190). Zinc deficiency also compromises adaptive immune function, including lymphocyte number and function (191). T lymphocytes (T cells) are particularly vulnerable to zinc deficiency (reviewed in 192). Zinc deficiency causes thymic atrophy, which leads to low numbers of T cells, and creates an imbalance in T helper cell subsets, with a shift towards Th2. Additionally, altered cytokine production during zinc deficiency can contribute to oxidative stress and inflammation (193, 194; reviewed in 195).
Even marginal zinc deficiency, which is more common than severe zinc deficiency, can suppress aspects of immunity (187). The elderly may be particularly at risk for marginal zinc deficiency given that there is a high prevalence of inadequate dietary zinc intake among those 60 years of age and older (196, 197) and that plasma zinc concentration declines with age (198, 199). It is not known why plasma zinc declines, but impaired absorption, alterations in cellular uptake, and epigenetic dysregulation may be contributing factors (reviewed in 200). Several randomized controlled trials demonstrate that supplementation with low to moderate doses of zinc (ranging from 10 to 45 mg zinc/day) in healthy elderly individuals improves several aspects of immune function, such as restoration of thymulin activity, increased numbers of cytotoxic T lymphocytes, reduced numbers of activated T helper cells (which can contribute to autoimmunity), increased NK cell cytotoxicity, and reduced incidence of infections (193, 201-205). For more information on zinc supplementation and the susceptibility to infectious diseases, such as diarrhea, pneumonia, and malaria, see the article on Zinc.
Adequate selenium intake is essential for the host to mount a proper immune response because it is required for the function of several selenium-dependent enzymes known as selenoproteins (see the article on Selenium). For example, the glutathione peroxidases (GPx) are selenoproteins that function as important redox regulators and cellular antioxidants, which reduce potentially damaging reactive oxygen species, such as hydrogen peroxide and lipid hydroperoxides, to harmless products like water and alcohols by coupling their reduction with the oxidation of glutathione (see Figure 2 in the article on selenium) (206). These roles have implications for immune function and cancer prevention.
Selenium deficiency impairs aspects of innate and adaptive immunity (207, 208), adversely affecting both humoral immunity (i.e., antibody production) and cell-mediated immunity (209). Selenium deficiency also appears to enhance the virulence or progression of some viral infections (see the article on Selenium) (210-212). Selenium affects different types of immune responses in different ways, and the selenium status of the host is an important factor when considering selenium supplementation (213). For example, selenium supplementation improves cell-mediated immunity in deficient individuals and enhances the immune response to viruses; on the other hand, selenium supplementation may worsen allergic asthma and impair the immune response to parasites (213, 214). A considerable amount of basic research also indicates that selenium plays a role in regulating the production of cytokines and eicosanoids that orchestrate the immune response (215, 216).
Iron is an essential component of hundreds of proteins and enzymes that are involved in oxygen transport and storage, electron transport and energy generation, antioxidant and beneficial pro-oxidant functions, and DNA synthesis (see the article on Iron) (217-219). Iron is required by the host in order to mount effective immune responses to invading pathogens, and iron deficiency impairs immune responses (220). Sufficient iron is critical to several immune functions, including the differentiation and proliferation of T lymphocytes and generation of reactive oxygen species (ROS) that kill pathogens. However, iron is also required by most infectious agents for replication and survival. During an acute inflammatory response, serum iron levels decrease while levels of ferritin (the iron storage protein) increase, suggesting that sequestering iron from pathogens is an important host response to infection (218, 221, 222). Moreover, conditions of iron overload (e.g., hereditary hemochromatosis) can have detrimental consequences to immune function, such as impairments in phagocytic function, cytokine production, complement system activation, and T and B lymphocyte function (see the article on Iron) (220, 223, 224).
Concern that iron administration could increase susceptibility to malaria and the risk of adverse events has received a lot of well-deserved attention (225). A large, randomized controlled trial (RCT) in Pemba, Zanzibar, where malaria is endemic, reported an increase in serious adverse events (hospital admissions and deaths) among young children (ages, 1-35 months) receiving iron plus folic acid supplements compared to those receiving placebo [RR: 1.12, 95% CI: 1.02-1.23]; notably, a substudy within the main Pemba trial determined that the detrimental effect was confined to children who were iron-replete at baseline (226). A 2011 Cochrane review found that oral iron supplementation alone did not influence the risk of clinical malaria (fever plus parasitemia) (13 trials), death (13 trials), or hospital admissions (4 trials) in malaria-endemic areas (227). However, a subgroup analysis revealed that the risk for clinical malaria was higher with iron supplementation in settings that lacked malaria surveillance and treatment [RR: 1.16, 95% CI: 1.03 to 1.31], suggesting that access to malaria diagnostic and treatment services is a critical component of iron supplementation efforts in malaria-endemic regions.
With respect to other infections, the Cochrane review found no beneficial or adverse effects of iron supplementation on respiratory tract infections (11 RCTs) or diarrheal episodes (13 RCTs) (227).
Copper is a critical functional component of a number of essential enzymes known as cuproenzymes (see the article on Copper). The mineral plays an important role in the development and maintenance of immune system function, but the exact mechanism of its action is not yet known. Copper has antimicrobial properties, accumulates at sites of inflammation, and may play a role in the innate immune response to bacterial infections (reviewed in 228). Copper deficiency results in neutropenia, an abnormally low number of neutrophils (229), which may increase one’s susceptibility to infection. Adverse effects of insufficient copper on immune function appear most pronounced in infants. Infants with Menkes disease, a genetic disorder that results in severe copper deficiency, suffer from frequent and severe infections (230, 231). In a study of 11 malnourished infants with evidence of copper deficiency, the ability of certain white blood cells to engulf pathogens increased significantly after one month of copper supplementation (232).
Immune effects have also been observed in adults with low intake of dietary copper. In one study, 11 men on a low-copper diet (0.66 mg copper/day for 24 days and 0.38 mg/day for another 40 days) showed a reduced proliferation response when white blood cells, called mononuclear cells, were isolated from blood and presented with an immune challenge in cell culture (233). While it is known that severe copper deficiency has adverse effects on immune function, the effects of marginal copper deficiency in humans are not yet clear (234). However, long-term high intakes of copper can result in adverse effects on immune function. In a small feeding study conducted in nine healthy young men, long-term high intake of copper (7.8 mg/day for approximately 5 months) blunted antibody production in response to an influenza vaccine (235).
Probiotics are usually defined as live microorganisms that, when administered in sufficient amounts, benefit the overall health of the host (236). Common examples belong to the Lactobacilli and Bifidobacteria species; these probiotics are consumed in yogurt and other fermented foods. Ingested probiotics that survive digestion can transiently inhabit the lower part of the gastrointestinal tract (237). Here, they can modulate immune functions by interacting with various receptors on intestinal epithelial cells and other gut-associated immune cells, including dendritic cells and M-cells (238). Immune modulation requires regular consumption because probiotics have not been shown to permanently alter intestinal microflora (239).
Probiotics have been shown to benefit both innate and adaptive immune responses of the host (240). Proposed mechanisms by which probiotics may influence immunity include the maintenance of the antimicrobial barrier, production of metabolic products that inhibit the growth of pathogens and influence host immune cell activity, and competition with pathogenic bacteria for available resources (13, 241). For example, probiotics can strengthen the gut epithelial barrier — an important innate defense — through a number of ways, such as by inhibiting apoptosis and promoting the survival of intestinal epithelial cells (242). Probiotics can also stimulate the production of antibodies and T lymphocytes, which are critical in the adaptive immune response (240). Several immune effects of probiotics are mediated through altering cell-signaling cascades that modify cytokine and other protein expression (242). However, probiotics exert diverse effects on the immune system that are dependent not only on the specific strain but also on the dose, route, and frequency of delivery (243).
A 2013 Cochrane review of 23 randomized controlled trials (RCTs) reported that probiotics reduce the risk of Clostridium difficile-associated diarrhea (CDAD) by 64% (95% CI: 26 to 51%) in participants taking antibiotics (244). Specifically, the incidence of CDAD in the probiotic group was 2.0% compared to 5.5% in the placebo or no treatment control group. This effect applied to both children and adults and was evident regardless of the dose or strain of administered probiotic. Another 2013 systematic review and meta-analysis, which focused on hospitalized adults (ages 33 to 79 years), reported a significant reduction in antibiotic-associated diarrhea (RR: 0.61, 95% CI 0.47 to 0.79) and Clostridium difficile infection (RR: 0.37, 95% CI: 0.22 to 0.61) in adults randomly assigned to co-administration of probiotics with antibiotics compared to co-administration of placebo with antibiotics (245). At this time, the scientific evidence is too weak to advocate the use of probiotics to reduce respiratory infections and improve vaccination response, especially in the elderly (reviewed in 246, 247). Probiotics may have utility in the prevention of inflammatory bowel disorders, allergic diseases, gastrointestinal and other types of infections, and certain cancers, but the clinical efficacy of probiotic administration for these applications is unknown at this time (240).
Overnutrition is a form of malnutrition where nutrients are supplied in excess of the body’s needs. Overnutrition can create an imbalance between energy intake and energy expenditure and lead to excessive energy storage, resulting in obesity (15). Obesity is a major public health problem worldwide, especially in industrialized nations. Obese individuals are at increased risk of morbidity from a number of chronic diseases, including hypertension and cardiovascular disease, type 2 diabetes mellitus, liver and gallbladder disease, osteoarthritis, sleep apnea, and certain cancers (248). Obesity has also been linked to increased risk of mortality (249).
Overnutrition and obesity have been shown to alter immunocompetence. Obesity is associated with macrophage infiltration of adipose tissue; macrophage accumulation in adipose tissue is directly proportional to the degree of obesity (250). Studies in mouse models of genetic and high-fat diet-induced obesity have documented a marked upregulation in expression of inflammation and macrophage-specific genes in white adipose tissue (251). In fact, obesity is characterized by chronic, low-grade inflammation, and inflammation is thought to be an important contributor in the pathogenesis of insulin resistance — a condition that is strongly linked to obesity. A number of changes occur in hypertrophied adipose tissue that might contribute to this inflammatory state: (1) altered adipokine secretion, namely increased leptin (pro-inflammatory) and reduced adiponectin (anti-inflammatory) secretion; (2) release of free fatty acids that induce systemic inflammation; (3) increased endoplasmic reticulum stress (leading to oxidative stress and inflammation) triggered by adipocyte expansion; and (4) increased inflammatory gene expression and immune cell activation in hypoxic regions (252). Alterations in the numbers, proportions, and activity of resident adipose tissue immune cells also help explain the pro-inflammatory phenotype of obesity (253, 254). Notably, visceral fat accumulation (central adiposity) appears to be more strongly associated with inflammation and adverse metabolic risk factors than subcutaneous fat, displaying increased macrophage infiltration, lower adiponectin gene expression, and increased inflammatory gene expression (252, 253).
Adipose tissue secretes fatty acids and other molecules, including various hormones and cytokines (called adipocytokines or adipokines), that trigger inflammatory processes (250). Leptin is one such hormone and adipokine that plays a key role in the regulation of food intake, body weight, and energy homeostasis (255, 256). Leptin is secreted from adipose tissue and circulates in direct proportion to the amount of fat stores. Normally, higher levels of circulating leptin suppress appetite and thereby lead to a reduction in food intake (257). Obese individuals have been reported to have higher plasma leptin concentrations compared to lean individuals. However, in the obese, the elevated leptin signal is not associated with the normal responses of reduced food intake and increased energy expenditure, suggesting obesity is associated with a state of leptin resistance. Leptin resistance has been documented in mouse models of obesity, but more research is needed to better understand leptin resistance in human obesity (257). Leptin has a number of other functions as well, such as modulation of inflammatory responses and aspects of humoral and cell-mediated responses of the adaptive immune system (255, 258). Specific effects of leptin, elucidated in animal and in vitro studies, include the promotion of phagocytic function of immune cells; stimulation of pro-inflammatory cytokine production; and regulation of neutrophil, natural killer (NK) cell, and dendritic cell functions (reviewed in 258). Leptin also affects aspects of cell-mediated immunity; for example, leptin promotes T helper (Th)1 immune responses and thus may have implications in the development of autoimmune disease (259). Th1 cells are primarily involved in activating macrophages and inflammatory responses (4, 257).
Obese individuals may exhibit increased susceptibility to various infections. Some epidemiological studies have shown that obese patients have a higher incidence of postoperative and other nosocomial infections compared with patients of normal weight (260, 261; reviewed in 262). Obesity has been linked to poor wound healing and increased occurrence of skin infections (263-265). A higher body mass index (BMI) may also be associated with increased susceptibility to respiratory, gastrointestinal, liver, and biliary infections (reviewed in 262). Several epidemiological studies have reported obesity to be an independent risk factor for increased morbidity and mortality following infection with the 2009 influenza A (H1N1) virus (266). In obesity, the increased vulnerability, severity, or complications of certain infections may be related to a number of factors, such as select micronutrient deficiencies. For example, one study in obese children and adolescents associated impairments in cell-mediated immunity with deficiencies in zinc and iron (267). Deficiencies or inadequacies of other vitamins, including the B vitamins and vitamins A, C, D, and E, have also been associated with obesity (52). Overall, immune responses appear to be compromised in obesity, but more research is needed to clarify the relationship between obesity and infection-related morbidity and mortality (reviewed in 266).
Written in August 2010 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in October 2015 by:
Giana Angelo, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in July 2016 by:
Adrian F. Gombart, Ph.D.
Associate Professor
Department of Biochemistry and Biophysics
Principal Investigator, Linus Pauling Institute
Oregon State University
The 2015 update of this article was underwritten, in part, by a grant from Bayer Consumer Care AG, Basel, Switzerland.
Copyright 2010-2024 Linus Pauling Institute
1. Nye KE. The basics of immunology for the non-immunologist. In: Hughes DA, Darlington LG, Bendich A, eds. Diet and Human Immune Function. Totowa, New Jersey: Humana Press; 2004:3-15.
2. Devereux G. The immune system: an overview. In: Calder PC, Field CJ, Gill HS, eds. Nutrition and Immune Function. New York: CABI Publishing; 2002:1-20.
3. The immune system. In: Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD, eds. Molecular Biology of the Cell. 3rd ed. New York: Garland Publishing, Inc.; 1994:1195-1254.
4. Parham P. T cell-mediated immunity. The Immune System. 2nd ed. New York: Garland Science Publishing; 2005:145-178.
5. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777-1789. (PubMed)
6. Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. 2000;343(1):37-49. (PubMed)
7. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 20(1):34-50. (PubMed)
8. Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140-1144. (PubMed)
9. Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058-1066. (PubMed)
10. Mak TW, Saunders ME. Innate immunity. The Immune Response: Basic and Clinical Principles. Amsterdam: Elsevier Academic Press; 2004:70-92.
11. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010; 327(5963):291-295. (PubMed)
12. Kohl J. Self, non-self, and danger: a complementary view. Adv Exp Med Biol. 2006;586:71-94. (PubMed)
13. Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72(3):299-309. (PubMed)
14. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582-1588. (PubMed)
15. Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115. (PubMed)
16. Food and Agriculture Organization of the United Nations. The state of food insecurity in the world 2006: eradicating world hunger--taking stock ten years after the World Food Summit. Available at: http://www.fao.org/docrep/009/a0750e/a0750e00.htm. Accessed 4/1/2010.
17. Chapman IM. Nutritional disorders in the elderly. Med Clin North Am. 2006;90(5):887-907. (PubMed)
18. Powell J, Borchers AT, Yoshida S, Gershwin ME. Evaluation of the immune system in the nutritionally at-risk host. In: Gershwin ME, German JB, Keen CL, eds. Nutrition and Immunology: Principles and Practice. Totowa, New Jersey: Humana Press; 2000:21-31.
19. Thurnham DI, Northrop-Clewes CA. Effects of infection on nutritional and immune status. In: Hughes DA, Darlington LG, Bendich A, eds. Diet and Human Immune Function. Totowa, New Jersey: Humana Press; 2004:35-64.
20. Jolly CA, Fernandes G. Protein-energy malnutrition and infectious disease. In: Gershwin ME, German JB, Keen CL, eds. Nutrition and Immunology: principles and practice. Totowa, New Jersey: Humana Press; 2000:195-202.
21. Batool R, Butt MS, Sultan MT, Saeed F, Naz R. Protein-energy malnutrition: a risk factor for various ailments. Crit Rev Food Sci Nutr. 2015;55(2):242-253. (PubMed)
22. de Onis M, Monteiro C, Akre J, Glugston G. The worldwide magnitude of protein-energy malnutrition: an overview from the WHO Global Database on Child Growth. Bull World Health Organ. 1993;71(6):703-712. (PubMed)
23. Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115(6):1119-1128; quiz 1129. (PubMed)
24. Grover Z, Ee LC. Protein energy malnutrition. Pediatr Clin North Am. 2009;56(5):1055-1068. (PubMed)
25. Sanchez Garcia E, Montero Errasquin B, Sanchez Castellano C, Cruz-Jentoft AJ. Importance of nutritional support in older people. Nestle Nutrition Institute Workshop Series. 2012;72:101-108.
26. Akner G, Cederholm T. Treatment of protein-energy malnutrition in chronic nonmalignant disorders. Am J Clin Nutr. 2001;74(1):6-24. (PubMed)
27. Chandra RK. Effect of post-natal protein malnutrition and intrauterine growth retardation on immunity and risk of infection. In: Calder PC, Field CJ, Gill HS, eds. Nutrition and Immune Function. New York: CABI Publishing; 2002:41-56.
28. Chandra RK. Protein-energy malnutrition and immunological responses. J Nutr. 1992;122(3 Suppl):597-600. (PubMed)
29. de Pablo MA, Puertollano MA, de Cienfuegos GA. Immune cell functions, lipids and host natural resistance. FEMS Immunol Med Microbiol. 2000;29:323-328.
30. Food and Nutrition Board, Institute of Medicine. Dietary fats: total fats and fatty acids. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty Acids, cholesterol, protein, and amino acids. Washington, D.C: The National Academies Press; 2002:422-541. (The National Academies Press)
31. Cunnane SC. Problems with essential fatty acids: time for a new paradigm? Prog Lipid Res. 2003;42(6):544-568. (PubMed)
32. Muskiet FA, Fokkema MR, Schaafsma A, Boersma ER, Crawford MA. Is docosahexaenoic acid (DHA) essential? Lessons from DHA status regulation, our ancient diet, epidemiology and randomized controlled trials. J Nutr. 2004;134(1):183-186. (PubMed)
33. Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55(1-3):123-139. (PubMed)
34. Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3-5):101-108. (PubMed)
35. Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72(3):326-336. (PubMed)
36. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197-202. (PubMed)
37. Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61(3):345-358. (PubMed)
38. Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38(4):323-341. (PubMed)
39. Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot Essent Fatty Acids. 2013;89(6):379-390. (PubMed)
40. Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73(3):539-548. (PubMed)
41. Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131(7):1918-1927. (PubMed)
42. Thies F, Miles EA, Nebe-von-Caron G, et al. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36(11):1183-1193. (PubMed)
43. Miles EA, Banerjee T, Wells SJ, Calder PC. Limited effect of eicosapentaenoic acid on T-lymphocyte and natural killer cell numbers and functions in healthy young males. Nutrition. 2006;22(5):512-519. (PubMed)
44. Rees D, Miles EA, Banerjee T, et al. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83(2):331-342. (PubMed)
45. O'Shea M, Bassaganya-Riera J, Mohede IC. Immunomodulatory properties of conjugated linoleic acid. Am J Clin Nutr. 2004;79(6 Suppl):1199S-1206S. (PubMed)
46. Song HJ, Grant I, Rotondo D, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr. 2005;59(4):508-517. (PubMed)
47. Akahoshi A, Goto Y, Murao K, et al. Conjugated linoleic acid reduces body fats and cytokine levels of mice. Biosci Biotechnol Biochem. 2002;66(4):916-920. (PubMed)
48. Sugano M, Tsujita A, Yamasaki M, Noguchi M, Yamada K. Conjugated linoleic acid modulates tissue levels of chemical mediators and immunoglobulins in rats. Lipids. 1998;33(5):521-527. (PubMed)
49. Albers R, van der Wielen RP, Brink EJ, Hendriks HF, Dorovska-Taran VN, Mohede IC. Effects of cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid (CLA) isomers on immune function in healthy men. Eur J Clin Nutr. 2003;57(4):595-603. (PubMed)
50. Kelley DS, Simon VA, Taylor PC, et al. Dietary supplementation with conjugated linoleic acid increased its concentration in human peripheral blood mononuclear cells, but did not alter their function. Lipids. 2001;36(7):669-674. (PubMed)
51. Kelley DS, Taylor PC, Rudolph IL, et al. Dietary conjugated linoleic acid did not alter immune status in young healthy women. Lipids. 2000;35(10):1065-1071. (PubMed)
52. Garcia OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67(10):559-572. (PubMed)
53. Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci U S A. 2006;103(47):17589-17594. (PubMed)
54. Moshfegh A, Goldman J, Cleveland L. 2005. What We Eat in America, NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. U.S. Department of Agriculture, Agricultural Research Service. Available at: http://www.ars.usda.gov/Services/docs.htm?docid=13793. Accessed 8/5/16.
55. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. (PubMed)
56. Semba RD. Vitamin A. In: Hughes DA, Darlington LG, Bendich A, eds. Diet and Human Immune Function. Totowa, New Jersey: Humana Press Inc.; 2004:105-131.
57. Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10(9):940-954. (PubMed)
58. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43(11):1773-1808. (PubMed)
59. Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2001;85 Suppl 2:S75-80. (PubMed)
60. Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167-192. (PubMed)
61. McDowell EM, Keenan KP, Huang M. Effects of vitamin A-deprivation on hamster tracheal epithelium. A quantitative morphologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):197-219. (PubMed)
62. Hatchell DL, Sommer A. Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol. 1984;102(9):1389-1393. (PubMed)
63. Barreto ML, Santos LM, Assis AM, et al. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet. 1994;344(8917):228-231. (PubMed)
64. Brown KH, Gaffar A, Alamgir SM. Xerophthalmia, protein-calorie malnutrition, and infections in children. J Pediatr. 1979;95(4):651-656. (PubMed)
65. Twining SS, Zhou X, Schulte DP, Wilson PM, Fish B, Moulder J. Effect of vitamin A deficiency on the early response to experimental Pseudomonas keratitis. Invest Ophthalmol Vis Sci. 1996;37(4):511-522. (PubMed)
66. Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr. 1984;40(5):1090-1095. (PubMed)
67. Rojanapo W, Lamb AJ, Olson JA. The prevalence, metabolism and migration of goblet cells in rat intestine following the induction of rapid, synchronous vitamin A deficiency. J Nutr. 1980;110(1):178-188. (PubMed)
68. Buck J, Derguini F, Levi E, Nakanishi K, Hammerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991;254(5038):1654-1656. (PubMed)
69. Blomhoff HK, Smeland EB, Erikstein B, et al. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem. 1992;267(33):23988-23992. (PubMed)
70. Pasatiempo AM, Bowman TA, Taylor CE, Ross AC. Vitamin A depletion and repletion: effects on antibody response to the capsular polysaccharide of Streptococcus pneumoniae, type III (SSS-III). Am J Clin Nutr. 1989;49(3):501-510. (PubMed)
71. Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80(4):581-586. (PubMed)
72. Smith SM, Hayes CE. Contrasting impairments in IgM and IgG responses of vitamin A-deficient mice. Proc Natl Acad Sci U S A. 1987;84(16):5878-5882. (PubMed)
73. Garbe A, Buck J, Hammerling U. Retinoids are important cofactors in T cell activation. J Exp Med. 1992;176(1):109-117. (PubMed)
74. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. (PubMed)
75. Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA. 1993;269(7):898-903. (PubMed)
76. Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis. 2000;182 Suppl 1:S122-133. (PubMed)
77. Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11 Suppl 3:S20. (PubMed)
78. Thorne-Lyman A, Fawzi WW. Vitamin A supplementation, infectious disease and child mortality: a summary of the evidence. Nestle Nutr Inst Workshop Ser. 2012;70:79-90. (PubMed)
79. Stephensen CB, Franchi LM, Hernandez H, Campos M, Gilman RH, Alvarez JO. Adverse effects of high-dose vitamin A supplements in children hospitalized with pneumonia. Pediatrics. 1998;101(5):E3. (PubMed)
80. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. The Vitamin A and Pneumonia Working Group. Bull World Health Organ. 1995;73(5):609-619. (PubMed)
81. Aukrust P, Muller F, Ueland T, Svardal AM, Berge RK, Froland SS. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest. 2000;30(3):252-259. (PubMed)
82. Sutton AL, MacDonald PN. Vitamin D: more than a "bone-a-fide" hormone. Mol Endocrinol. 2003;17(5):777-791. (PubMed)
83. Grober U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol. 2013;5(3):331-347. (PubMed)
84. Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61(4):457-461. (PubMed)
85. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181-1183. (PubMed)
86. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334-338. (PubMed)
87. Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308-1310. (PubMed)
88. Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215(1-2):31-38. (PubMed)
89. Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12(4):388-390. (PubMed)
90. Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151-1165. (PubMed)
91. Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080-1082. (PubMed)
92. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19(9):1067-1077. (PubMed)
93. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770-1773. (PubMed)
94. Campbell Y, Fantacone ML, Gombart AF. Regulation of antimicrobial peptide gene expression by nutrients and by-products of microbial metabolism. Eur J Nutr. 2012;51(8):899-907. (PubMed)
95. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685-698. (PubMed)
96. Chen WC, Vayuvegula B, Gupta S. 1,25-Dihydroxyvitamin D3-mediated inhibition of human B cell differentiation. Clin Exp Immunol. 1987;69(3):639-646. (PubMed)
97. Muller K, Heilmann C, Poulsen LK, Barington T, Bendtzen K. The role of monocytes and T cells in 1,25-dihydroxyvitamin D3 mediated inhibition of B cell function in vitro. Immunopharmacology. 1991;21(2):121-128. (PubMed)
98. Muller K, Odum N, Bendtzen K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett. 1993;35(2):177-182. (PubMed)
99. Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6 Suppl):1704S-1708S. (PubMed)
100. Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8(4):174-179. (PubMed)
101. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974-4980. (PubMed)
102. Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160(11):5314-5319. (PubMed)
103. Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51(5):1367-1374. (PubMed)
104. Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195(5):603-616. (PubMed)
105. Baeke F, Etten EV, Overbergh L, Mathieu C. Vitamin D3 and the immune system: maintaining the balance in health and disease. Nutr Res Rev. 2007;20(1):106-118. (PubMed)
106. Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. Faseb J. 2001;15(14):2579-2585. (PubMed)
107. Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol. 2013;45(2):256-266. (PubMed)
108. Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12(2):127-136. (PubMed)
109. Duan S, Lv Z, Fan X, et al. Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. Neurosci Lett. 2014;570:108-113. (PubMed)
110. Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45(2):217-226. (PubMed)
111. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229(11):1136-1142. (PubMed)
112. Simpson S, Jr., Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1132-1141. (PubMed)
113. Munger KL, Ascherio A. Prevention and treatment of MS: studying the effects of vitamin D. Mult Scler. 2011;17(12):1405-1411. (PubMed)
114. Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5(9):3551-3562. (PubMed)
115. Song GG, Bae SC, Lee YH. Association between vitamin D intake and the risk of rheumatoid arthritis: a meta-analysis. Clin Rheumatol. 2012;31(12):1733-1739. (PubMed)
116. Hyppönen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500-1503. (PubMed)
117. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838. (PubMed)
118. Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60-65. (PubMed)
119. Ozgocmen S, Bulut S, Ilhan N, Gulkesen A, Ardicoglu O, Ozkan Y. Vitamin D deficiency and reduced bone mineral density in multiple sclerosis: effect of ambulatory status and functional capacity. J Bone Miner Metab. 2005;23(4):309-313. (PubMed)
120. van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254(5):581-590. (PubMed)
121. Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50(1):72-77. (PubMed)
122. James E, Dobson R, Kuhle J, Baker D, Giovannoni G, Ramagopalan SV. The effect of vitamin D-related interventions on multiple sclerosis relapses: a meta-analysis. Mult Scler. 2013;19(12):1571-1579. (PubMed)
123. Pozuelo-Moyano B, Benito-Leon J, Mitchell AJ, Hernandez-Gallego J. A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis. Neuroepidemiology. 2013;40(3):147-153. (PubMed)
124. Bhargava P, Cassard S, Steele SU, et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials. 2014;39(2):288-293. (PubMed)
125. Smolders J, Hupperts R, Barkhof F, et al. Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011;311(1-2):44-49. (PubMed)
126. Dörr J, Ohlraun S, Skarabis H, Paul F. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS Trial): study protocol for a randomized controlled trial. Trials. 2012;13:15. (PubMed)
127. Prinz W, Bortz R, Bregin B, Hersch M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res. 1977;47(3):248-257. (PubMed)
128. Vallance S. Relationships between ascorbic acid and serum proteins of the immune system. Br Med J. 1977;2(6084):437-438. (PubMed)
129. Kennes B, Dumont I, Brohee D, Hubert C, Neve P. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology. 1983;29(5):305-310. (PubMed)
130. Panush RS, Delafuente JC, Katz P, Johnson J. Modulation of certain immunologic responses by vitamin C. III. Potentiation of in vitro and in vivo lymphocyte responses. Int J Vitam Nutr Res Suppl. 1982;23:35-47. (PubMed)
131. Jariwalla RJ, Harakeh S. Antiviral and immunomodulatory activities of ascorbic acid. In: Harris JR, ed. Subcellular Biochemistry. Ascorbic Acid: Biochemistry and Biomedical Cell Biology. New York: Plenum Press; 1996:215-231.
132. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980;33(1):71-76. (PubMed)
133. Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. Vitamin C for the treatment of recurrent furunculosis in patients with imparied neutrophil functions. J Infect Dis. 1996;173(6):1502-1505. (PubMed)
134. Anderson R. The immunostimulatory, antiinflammatory and anti-allergic properties of ascorbate. Adv Nutr Res. 1984;6:19-45. (PubMed)
135. Huijskens MJ, Walczak M, Koller N, et al. Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol. 2014;96(6):1165-1175. (PubMed)
136. Schwager J, Bompard A, Weber P, Raederstorff D. Ascorbic acid modulates cell migration in differentiated HL-60 cells and peripheral blood leukocytes. Mol Nutr Food Res. 2015;59(8):1513-1523. (PubMed)
137. Bozonet SM, Carr AC, Pullar JM, Vissers MC. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients. 2015;7(4):2574-2588. (PubMed)
138. Feigen GA, Smith BH, Dix CE, et al. Enhancement of antibody production and protection against systemic anaphylaxis by large doses of vitamin C. Res Commun Chem Pathol Pharmacol. 1982;38(2):313-333. (PubMed)
139. Prinz W, Bloch J, Gilich G, Mitchell G. A systematic study of the effect of vitamin C supplementation on the humoral immune response in ascorbate-dependent mammals. I. The antibody response to sheep red blood cells (a T-dependent antigen) in guinea pigs. Int J Vitam Nutr Res. 1980;50(3):294-300. (PubMed)
140. Haskell BE, Johnston CS. Complement component C1q activity and ascorbic acid nutriture in guinea pigs. Am J Clin Nutr. 1991;54(6 Suppl):1228S-1230S. (PubMed)
141. Johnston CS, Cartee GD, Haskell BE. Effect of ascorbic acid nutriture on protein-bound hydroxyproline in guinea pig plasma. J Nutr. 1985;115(8):1089-1093. (PubMed)
142. Johnston CS, Kolb WP, Haskell BE. The effect of vitamin C nutriture on complement component C1q concentrations in guinea pig plasma. J Nutr. 1987;117(4):764-768. (PubMed)
143. Shilotri PG, Bhat KS. Effect of mega doses of vitamin C on bactericidal ativity of leukocytes. Am J Clin Nutr. 1977;30(7):1077-1081. (PubMed)
144. Vogel RI, Lamster IB, Wechsler SA, Macedo B, Hartley LJ, Macedo JA. The effects of megadoses of ascorbic acid on PMN chemotaxis and experimental gingivitis. J Periodontol. 1986;57(8):472-479. (PubMed)
145. Ludvigsson J, Hansson LO, Stendahl O. The effect of large doses of vitamin C on leukocyte function and some laboratory parameters. Int J Vitam Nutr Res. 1979;49(2):160-165. (PubMed)
146. Delafuente JC, Prendergast JM, Modigh A. Immunologic modulation by vitamin C in the elderly. Int J Immunopharmacol. 1986;8(2):205-211. (PubMed)
147. Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem. 1990;265(5):2584-2587. (PubMed)
148. Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr. 1982;47(3):473-482. (PubMed)
149. Differentiated cells and the maintenance of tissues. In: Alberts B BD, Lewis J, Raff M, Roberts K, Watson JD, ed. Molecular Biology of the Cell. 3rd ed. New York: Garland Publishing, Inc.; 1994:1139-1193.
150. Jariwalla RJ, Harakeh S. Mechanisms underlying the action of vitamin C in viral and immunodeficiency disease. In: L. P, J. F, eds. Vitamin C in Health and Disease. New York: Macel Dekker, Inc.; 1997:309-322.
151. Pauling L. The immune system. How to Live Longer and Feel Better. 20th anniversary ed. Corvallis: Oregon State University Press; 2006:105-111.
152. Dahl H, Degré M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol Microbiol Scand B. 1976;84B(5):280-284. (PubMed)
153. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086-1107. (PubMed)
154. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;8:CD005532. (PubMed)
155. Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43(1):4-15. (PubMed)
156. Moriguchi S, Muraga M. Vitamin E and immunity. Vitam Horm. 2000;59:305-336. (PubMed)
157. Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283-289. (PubMed)
158. Molano A, Meydani SN. Vitamin E, signalosomes and gene expression in T cells. Mol Aspects Med. 2012;33(1):55-62. (PubMed)
159. De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res. 2008;42(3):272-280. (PubMed)
160. Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277(17):1380-1386. (PubMed)
161. Pallast EG, Schouten EG, de Waart FG, et al. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr. 1999;69(6):1273-1281. (PubMed)
162. Meydani SN, Leka LS, Fine BC, et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292(7):828-836. (PubMed)
163. Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211(2):144-156. (PubMed)
164. Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70-74. (PubMed)
165. Wang Y, Huang DS, Watson RR. Dietary vitamin E modulation of cytokine production by splenocytes and thymocytes from alcohol-fed mice. Alcohol Clin Exp Res. 1994;18(2):355-362. (PubMed)
166. Wang Y, Watson RR. Vitamin E supplementation at various levels alters cytokine production by thymocytes during retrovirus infection causing murine AIDS. Thymus. 1994;22(3):153-165. (PubMed)
167. Serafini M. Dietary vitamin E and T cell-mediated function in the elderly: effectiveness and mechanism of action. Int J Dev Neurosci. 2000;18(4-5):401-410. (PubMed)
168. Han SN, Meydani SN. Vitamin E and infectious diseases in the aged. Proc Nutr Soc. 1999;58(3):697-705. (PubMed)
169. Harman D, Miller RA. Effect of vitamin E on the immune response to influenza virus vaccine and the incidence of infectious disease in man. Age. 1986;9:21-23.
170. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA. 2002;288(6):715-721. (PubMed)
171. Hemilä H, Virtamo J, Albanes D, Kaprio J. The effect of vitamin E on common cold incidence is modified by age, smoking and residential neighborhood. J Am Coll Nutr. 2006;25(4):332-339. (PubMed)
172. Chandra RK, Sudhakaran L. Regulation of immune responses by vitamin B6. Ann N Y Acad Sci. 1990;585:404-423. (PubMed)
173. Trakatellis A, Dimitriadou A, Trakatelli M. Pyridoxine deficiency: new approaches in immunosuppression and chemotherapy. Postgrad Med J. 1997;73(864):617-622. (PubMed)
174. Rall LC, Meydani SN. Vitamin B6 and immune competence. Nutr Rev. 1993;51(8):217-225. (PubMed)
175. Paul L, Ueland PM, Selhub J. Mechanistic perspective on the relationship between pyridoxal 5'-phosphate and inflammation. Nutr Rev. 2013;71(4):239-244. (PubMed)
176. Meydani SN, Ribaya-Mercado JD, Russell RM, Sahyoun N, Morrow FD, Gershoff SN. Vitamin B-6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am J Clin Nutr. 1991;53(5):1275-1280. (PubMed)
177. Talbott MC, Miller LT, Kerkvliet NI. Pyridoxine supplementation: effect on lymphocyte responses in elderly persons. Am J Clin Nutr. 1987;46(4):659-664. (PubMed)
178. Bailey LB, Gregory JF, 3rd. Folate. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. 9th ed. Washington, D.C.: ILSI Press; 2006:278-301.
179. Bailey LB, Gregory JF, 3rd. Folate metabolism and requirements. J Nutr. 1999;129(4):779-782. (PubMed)
180. Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15(1-2):43-60. (PubMed)
181. Gross RL, Reid JV, Newberne PM, Burgess B, Marston R, Hift W. Depressed cell-mediated immunity in megaloblastic anemia due to folic acid deficiency. Am J Clin Nutr. 1975;28(3):225-232. (PubMed)
182. Shane B. Folic acid, vitamin B-12, and vitamin B-6. In: Stipanuk M, ed. Biochemical and Physiological Aspects of Human Nutrition. 2nd ed. Philadelphia: Saunders Elsevier; 2006:693-732.
183. Erkurt MA, Aydogdu I, Dikilitas M, et al. Effects of cyanocobalamin on immunity in patients with pernicious anemia. Med Princ Pract. 2008;17(2):131-135. (PubMed)
184. Tamura J, Kubota K, Murakami H, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28-32. (PubMed)
185. Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5-6):353-357. (PubMed)
186. Cousins RJ. Zinc. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. Washington, D.C.: ILSI Press; 2006:445-457.
187. Ibs K-H, Rink L. Zinc. In: Hughes DA, Darlington LG, Bendich A, eds. Diet and Human Immune Function. Totowa, New Jersey: Human Press Inc.; 2004:241-259.
188. Kruse-Jarres JD. The significance of zinc for humoral and cellular immunity. J Trace Elem Electrolytes Health Dis. 1989;3(1):1-8. (PubMed)
189. Allen JI, Perri RT, McClain CJ, Kay NE. Alterations in human natural killer cell activity and monocyte cytotoxicity induced by zinc deficiency. J Lab Clin Med. 1983;102(4):577-589. (PubMed)
190. Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 Suppl 1):1452S-1456S. (PubMed)
191. Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S-463S. (PubMed)
192. Bonaventura P, Benedetti G, Albarede F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277-285. (PubMed)
193. Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85(3):837-844. (PubMed)
194. Bao B, Prasad AS, Beck FW, et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. 2010;91(6):1634-1641. (PubMed)
195. Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676-694. (PubMed)
196. Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults in the 1994-96 continuing survey of food intakes by individuals (CSFII). J Nutr. 2000;130(11):2838-2843. (PubMed)
197. Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988-1994. J Nutr. 2000;130(5S Suppl):1367S-1373S. (PubMed)
198. Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr. 2003;78(4):756-764. (PubMed)
199. Ravaglia G, Forti P, Maioli F, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85(6):2260-2265. (PubMed)
200. Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res. 2012;56(1):77-87. (PubMed)
201. Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition. 1993;9(3):218-224. (PubMed)
202. Boukaiba N, Flament C, Acher S, et al. A physiological amount of zinc supplementation: effects on nutritional, lipid, and thymic status in an elderly population. Am J Clin Nutr. 1993;57(4):566-572. (PubMed)
203. Fortes C, Forastiere F, Agabiti N, et al. The effect of zinc and vitamin A supplementation on immune response in an older population. J Am Geriatr Soc. 1998;46(1):19-26. (PubMed)
204. Kahmann L, Uciechowski P, Warmuth S, et al. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008;11(1):227-237. (PubMed)
205. Mocchegiani E, Giacconi R, Costarelli L, et al. Zinc deficiency and IL-6 -174G/C polymorphism in old people from different European countries: effect of zinc supplementation. ZINCAGE study. Exp Gerontol. 2008;43(5):433-444. (PubMed)
206. Gladyshev VN. Selenoproteins and selenoproteomes. In: Hatfield DL, Berry MJ, Gladyshev VN, eds. Selenium: Its Molecular Biology and Role in Human Health. 2nd ed. New York: Springer; 2006:99-114.
207. Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003;133(5 Suppl 1):1457S-1459S. (PubMed)
208. McKenzie RC, Beckett GJ, Arthur JR. Effects of selenium on immunity and aging. In: Hatfield DL, Berry MJ, Gladyshev VN, eds. Selenium: Its Molecular Biology and Role in Human Health. 2nd ed. New York: Springer; 2006:311-323.
209. Spallholz JE, Boylan LM, Larsen HS. Advances in understanding selenium's role in the immune system. Ann N Y Acad Sci. 1990;587:123-139. (PubMed)
210. Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res. 1994;41(1-2):115-127. (PubMed)
211. Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace Elem Res. 1994;41(1-2):103-114. (PubMed)
212. Kiremidjian-Schumacher L, Roy M, Glickman R, et al. Selenium and immunocompetence in patients with head and neck cancer. Biol Trace Elem Res. 2000;73(2):97-111. (PubMed)
213. Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273-1280. (PubMed)
214. Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154-162. (PubMed)
215. Baum MK, Miguez-Burbano MJ, Campa A, Shor-Posner G. Selenium and interleukins in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;182 Suppl 1:S69-73. (PubMed)
216. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705-743. (PubMed)
217. Brody T. Nutritional Biochemistry. 2nd ed. San Diego: Academic Press; 1999.
218. Beard J. Iron. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition. 9th ed. Washington, D.C.: ILSI Press; 2006:430-444.
219. Wood RJ, Ronnenberg AG. Iron. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2006:248-270.
220. Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137(5):1341-1344. (PubMed)
221. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2S-2):568S-579S; discussion 580S. (PubMed)
222. Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509-519. (PubMed)
223. Prentice AM. Iron metabolism, malaria, and other infections: what is all the fuss about? J Nutr. 2008;138(12):2537-2541. (PubMed)
224. Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133-143. (PubMed)
225. Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011(10):CD006589. (PubMed)
226. Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287(17):13549-13555. (PubMed)
227. Williams DM. Copper deficiency in humans. Semin Hematol. 1983;20(2):118-128. (PubMed)
228. Percival SS. Copper and immunity. Am J Clin Nutr. 1998;67(5 Suppl):1064S-1068S. (PubMed)
229. Failla ML, Hopkins RG. Is low copper status immunosuppressive? Nutr Rev. 1998;56(1 Pt 2):S59-64. (PubMed)
230. Heresi G, Castillo-Duran C, Munoz C, Arevalo M, Schlesinger L. Phagocytosis and immunoglobulin levels in hypocupremic children. Nutr Res 1985;5:1327-1334.
231. Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62(2):412-416. (PubMed)
232. Bonham M, O'Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? Br J Nutr. 2002;87(5):393-403. (PubMed)
233. Turnlund JR, Jacob RA, Keen CL, et al. Long-term high copper intake: effects on indexes of copper status, antioxidant status, and immune function in young men. Am J Clin Nutr. 2004;79(6):1037-1044. (PubMed)
234. Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44(1):26-46. (PubMed)
235. Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71(6 Suppl):1682S-1687S; discussion 1688S-1690S. (PubMed)
236. West NP, Pyne DB, Peake JM, Cripps AW. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15:107-126. (PubMed)
237. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1-66. (PubMed)
238. Ruemmele FM, Bier D, Marteau P, et al. Clinical evidence for immunomodulatory effects of probiotic bacteria. J Pediatr Gastroenterol Nutr. 2009;48(2):126-141. (PubMed)
239. Vieira AT, Teixeira MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol. 2013;4:445. (PubMed)
240. Oelschlaeger TA. Mechanisms of probiotic actions - A review. Int J Med Microbiol.300(1):57-62. (PubMed)
241. Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract. 2009;24(1):10-14. (PubMed)
242. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2013;5:CD006095. (PubMed)
243. Pattani R, Palda VA, Hwang SW, Shah PS. Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile infection among hospitalized patients: systematic review and meta-analysis. Open Med. 2013;7(2):e56-67. (PubMed)
244. Yaqoob P. Ageing, immunity and influenza: a role for probiotics? Proc Nutr Soc. 2014;73(2):309-317. (PubMed)
245. Maidens C, Childs C, Przemska A, Dayel IB, Yaqoob P. Modulation of vaccine response by concomitant probiotic administration. Br J Clin Pharmacol. 2013;75(3):663-670. (PubMed)
246. National Institutes of Health NOEI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. September 1998. Available online at: http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf.
247. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW, Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097-1105. (PubMed)
248. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol.72:219-246. (PubMed)
249. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821-1830. (PubMed)
250. de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332-338. (PubMed)
251. Exley MA, Hand L, O'Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. J Endocrinol. 2014;223(2):R41-48. (PubMed)
252. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222(3):R113-127. (PubMed)
253. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174(6):3137-3142. (PubMed)
254. Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (Silver Spring). 18(2):221-229. (PubMed)
255. Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring). 2006;14 Suppl 5:254S-258S. (PubMed)
256. Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252(1-2):139-145. (PubMed)
257. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95. (PubMed)
258. Espejo B, Torres A, Valentin M, et al. Obesity favors surgical and infectious complications after renal transplantation. Transplant Proc. 2003;35(5):1762-1763. (PubMed)
259. Canturk Z, Canturk NZ, Cetinarslan B, Utkan NZ, Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11(6):769-775. (PubMed)
260. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438-446. (PubMed)
261. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17(8):426-435. (PubMed)
262. Scheinfeld NS. Obesity and dermatology. Clin Dermatol. 2004;22(4):303-309. (PubMed)
263. Janniger CK, Schwartz RA, Szepietowski JC, Reich A. Intertrigo and common secondary skin infections. Am Fam Physician. 2005;72(5):833-838. (PubMed)
264. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298-306. (PubMed)
265. Chandra RK, Kutty KM. Immunocompetence in obesity. Acta Paediatr Scand. 1980;69(1):25-30. (PubMed)
266. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298-306. (PubMed)
267. Chandra RK, Kutty KM. Immunocompetence in obesity. Acta Paediatr Scand. 1980;69(1):25-30. (PubMed)